Effect of Dapagliflozin as an Add-on Therapy to Insulin on the Glycemic Variability in Subjects with Type 2 Diabetes Mellitus (DIVE): A Multicenter, Placebo-Controlled, Double-Blind, Randomized Study
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Medical Informatics, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea.
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Endocrinology and Metabolism, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea.
- 6Division of Endocrinology and Metabolism, Department of Internal Medicine, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea.
- 7Division of Endocrinology and Metabolism, Department of Internal Medicine, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 8MedicalExcellence Inc., Seoul, Korea.
- KMID: 2516336
- DOI: http://doi.org/10.4093/dmj.2019.0203
Abstract
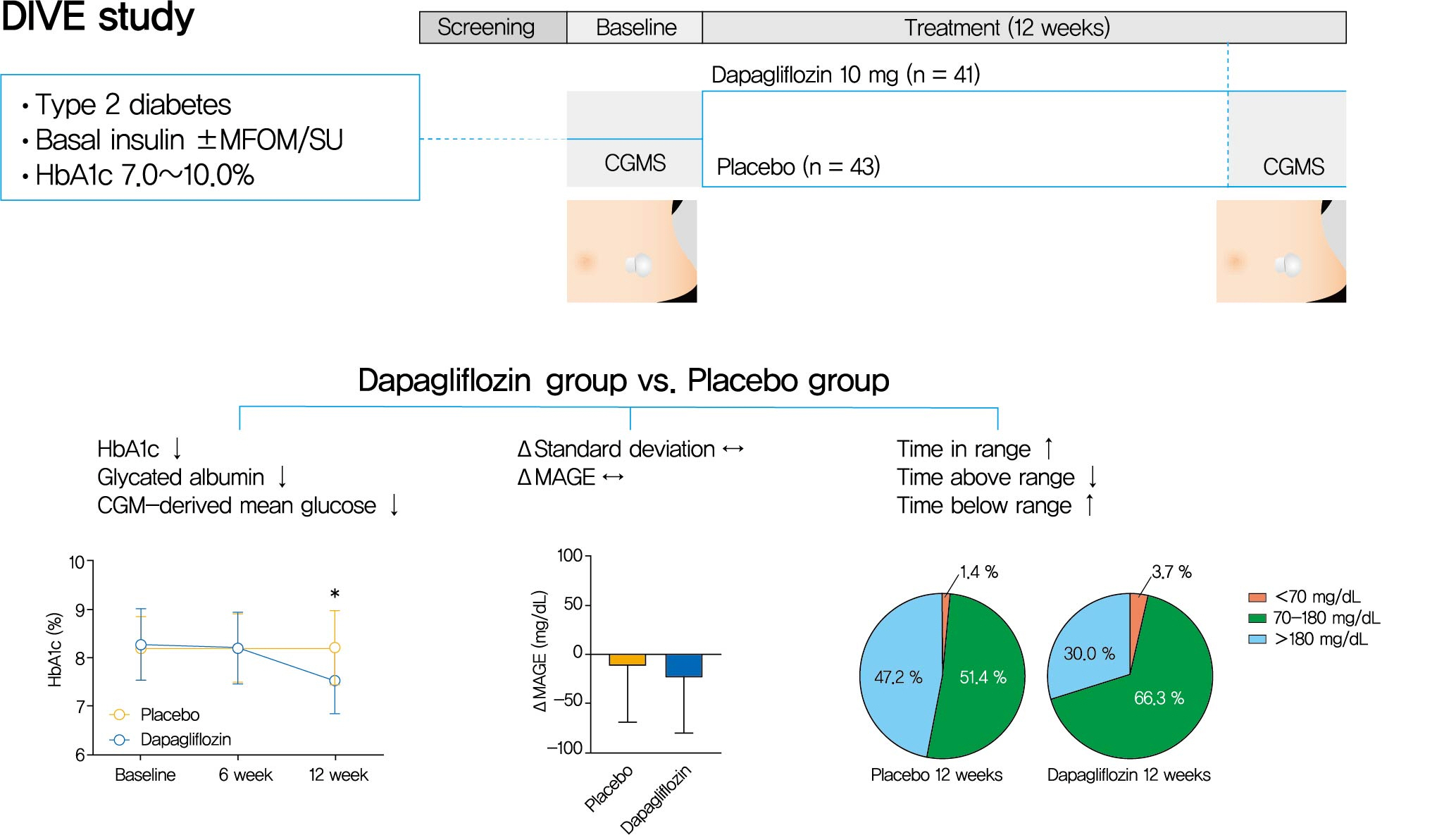
Background Glycemic variability is associated with the development of diabetic complications and hypoglycemia. However, the effect of sodium-glucose transporter 2 (SGLT2) inhibitors on glycemic variability is controversial. We aimed to examine the effect of dapagliflozin as an add-on therapy to insulin on the glycemic variability assessed using continuous glucose monitoring (CGM) in subjects with type 2 diabetes mellitus.
Methods In this multicenter, placebo-controlled, double-blind, randomized study, 84 subjects received 10 mg of dapagliflozin (
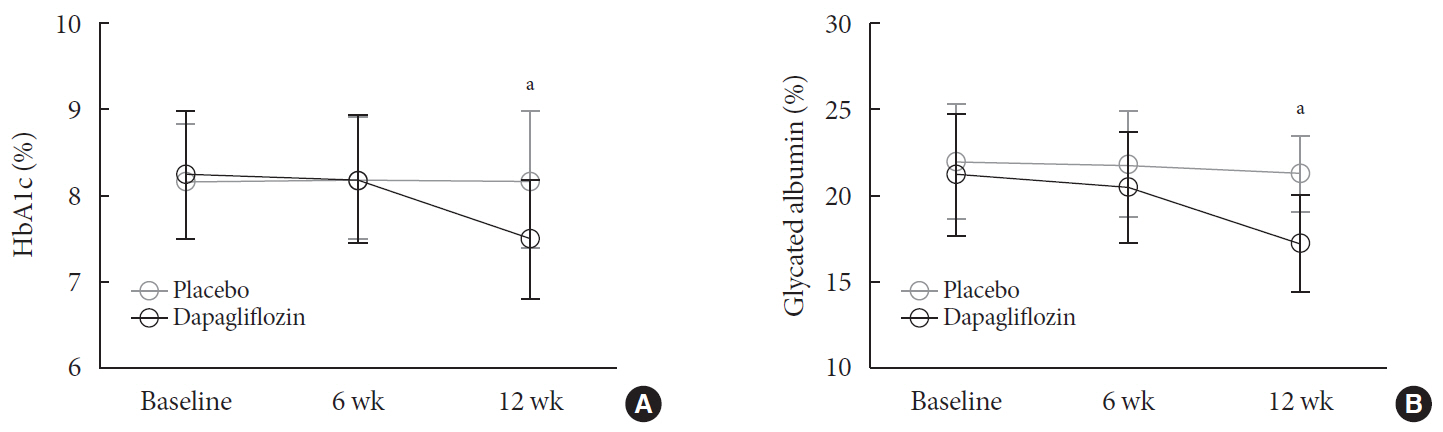
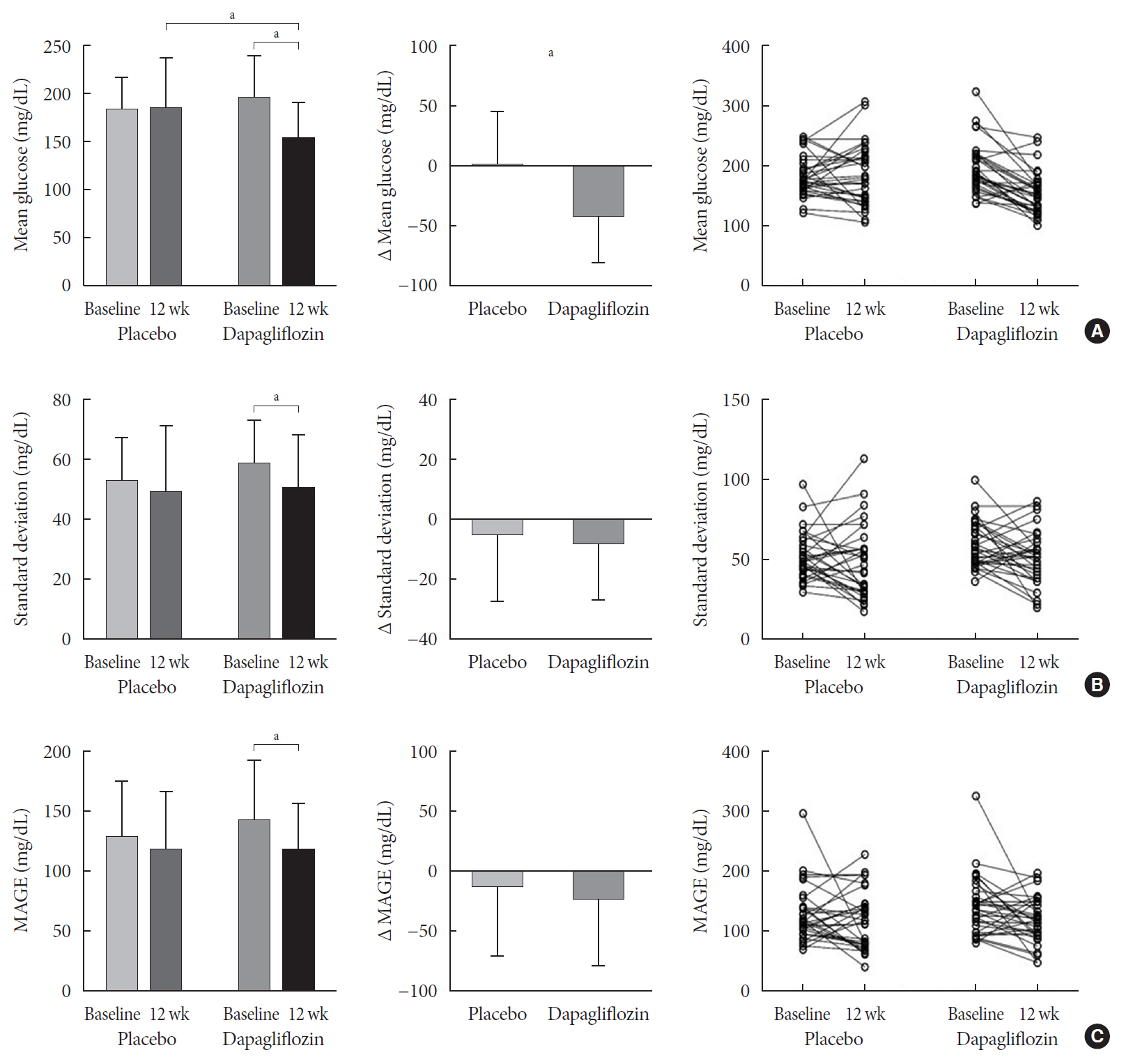
n =41) or the placebo (n =43) for 12 weeks. CGM was performed before and after treatment to compare the changes in glycemic variability measures (standard deviation [SD], mean amplitude of glycemic excursions [MAGEs]).Results At week 12, significant reductions in glycosylated hemoglobin (−0.74%±0.66% vs. 0.01%±0.65%,
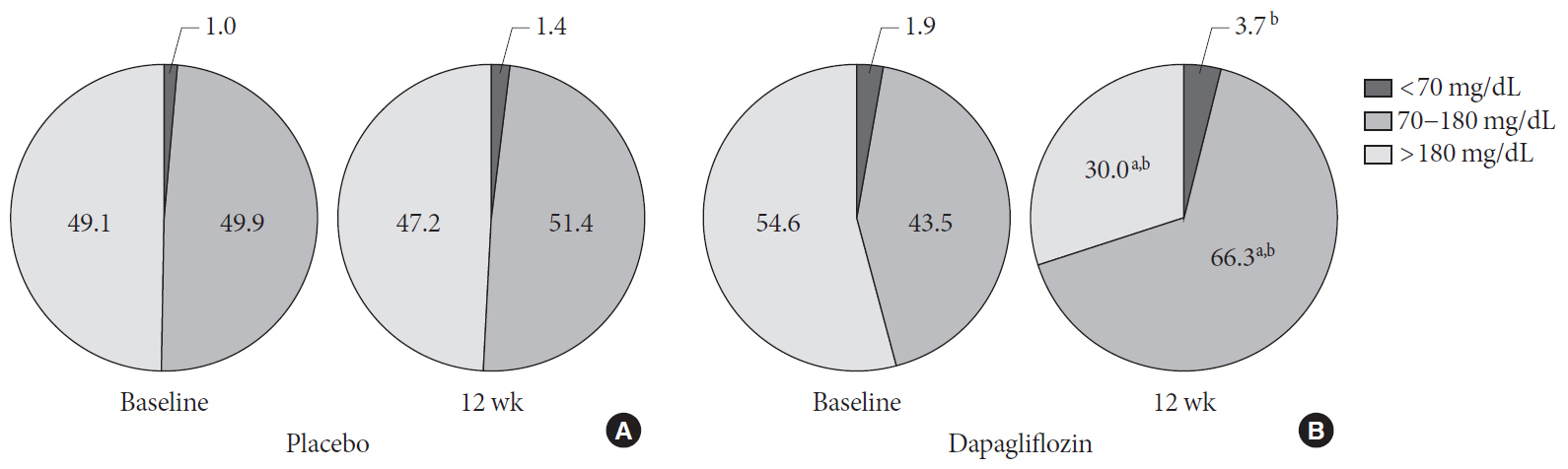
P <0.001), glycated albumin (−3.94%±2.55% vs. −0.67%±2.48%,P <0.001), and CGM-derived mean glucose (−41.6±39.2 mg/dL vs. 1.1±46.2 mg/dL,P <0.001) levels were observed in the dapagliflozin group compared with the placebo group. SD and MAGE were significantly decreased in the dapagliflozin group, but not in the placebo group. However, the difference in ΔSD and ΔMAGE failed to reach statistical significance between two groups. No significant differences in the incidence of safety endpoints were observed between the two groups.Conclusion Dapagliflozin effectively decreased glucose levels, but not glucose variability, after 12 weeks of treatment in participants with type 2 diabetes mellitus receiving insulin treatment. The role of SGLT2 inhibitors in glycemic variability warrants further investigations.
Keyword
Figure
Cited by 2 articles
-
Time in Range from Continuous Glucose Monitoring: A Novel Metric for Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes Metab J. 2020;44(6):828-839. doi: 10.4093/dmj.2020.0257.Association between Variability of Metabolic Risk Factors and Cardiometabolic Outcomes
Min Jeong Park, Kyung Mook Choi
Diabetes Metab J. 2022;46(1):49-62. doi: 10.4093/dmj.2021.0316.
Reference
-
1. Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015; 39:273–82.
Article2. Jung HS. Clinical implications of glucose variability: chronic complications of diabetes. Endocrinol Metab (Seoul). 2015; 30:167–74.
Article3. Umpierrez GE, P Kovatchev B. Glycemic variability: how to measure and its clinical implication for type 2 diabetes. Am J Med Sci. 2018; 356:518–27.
Article4. Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014; 37:2359–65.
Article5. Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015; 38:2354–69.
Article6. Hirsch IB. Glycemic variability and diabetes complications: does it matter? Of course it does! Diabetes Care. 2015; 38:1610–4.
Article7. Xu D, Fang H, Xu W, Yan Y, Liu Y, Yao B. Fasting plasma glucose variability and all-cause mortality among type 2 diabetes patients: a dynamic cohort study in Shanghai, China. Sci Rep. 2016; 6:39633.
Article8. Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol. 2018; 17:33.
Article9. Chao EC, Henry RR. SGLT2 inhibition: a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010; 9:551–9.10. DeFronzo RA, Hompesch M, Kasichayanula S, Liu X, Hong Y, Pfister M, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013; 36:3169–76.
Article11. Lee JY, Cho Y, Lee M, Kim YJ, Lee YH, Lee BW, et al. Predictors of the therapeutic efficacy and consideration of the best combination therapy of sodium-glucose co-transporter 2 inhibitors. Diabetes Metab J. 2019; 43:158–73.
Article12. Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018; 61:2079–86.
Article13. Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thevenet J, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015; 21:512–7.
Article14. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014; 124:509–14.
Article15. Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015; 38:412–9.
Article16. Nishimura R, Osonoi T, Kanada S, Jinnouchi H, Sugio K, Omiya H, et al. Effects of luseogliflozin, a sodium-glucose co-transporter 2 inhibitor, on 24-h glucose variability assessed by continuous glucose monitoring in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, crossover study. Diabetes Obes Metab. 2015; 17:800–4.17. Nishimura R, Tanaka Y, Koiwai K, Inoue K, Hach T, Salsali A, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015; 14:11.
Article18. Sands AT, Zambrowicz BP, Rosenstock J, Lapuerta P, Bode BW, Garg SK, et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care. 2015; 38:1181–8.
Article19. Nishimura R, Omiya H, Sugio K, Ubukata M, Sakai S, Samukawa Y. Sodium- glucose cotransporter 2 inhibitor luseogliflozin improves glycaemic control, assessed by continuous glucose monitoring, even on a low-carbohydrate diet. Diabetes Obes Metab. 2016; 18:702–6.20. Famulla S, Pieber TR, Eilbracht J, Neubacher D, Soleymanlou N, Woerle HJ, et al. Glucose exposure and variability with empagliflozin as adjunct to insulin in patients with type 1 diabetes: continuous glucose monitoring data from a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Technol Ther. 2017; 19:49–60.
Article21. Matsumura M, Nakatani Y, Tanka S, Aoki C, Sagara M, Yanagi K, et al. Efficacy of additional canagliflozin administration to type 2 diabetes patients receiving insulin therapy: examination of diurnal glycemic patterns using continuous glucose monitoring (CGM). Diabetes Ther. 2017; 8:821–7.
Article22. Nomoto H, Miyoshi H, Sugawara H, Ono K, Yanagiya S, Oita M, et al. A randomized controlled trial comparing the effects of dapagliflozin and DPP-4 inhibitors on glucose variability and metabolic parameters in patients with type 2 diabetes mellitus on insulin. Diabetol Metab Syndr. 2017; 9:54.
Article23. Rodbard HW, Peters AL, Slee A, Cao A, Traina SB, Alba M. The effect of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care. 2017; 40:171–80.
Article24. Henry RR, Strange P, Zhou R, Pettus J, Shi L, Zhuplatov SB, et al. Effects of dapagliflozin on 24-hour glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetes Technol Ther. 2018; 20:715–24.
Article25. Danne T, Cariou B, Buse JB, Garg SK, Rosenstock J, Banks P, et al. Improved time in range and glycemic variability with sotagliflozin in combination with insulin in adults with type 1 diabetes: a pooled analysis of 24-week continuous glucose monitoring data from the intandem program. Diabetes Care. 2019; 42:919–30.
Article26. Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970; 19:644–55.
Article27. Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001; 281:E924–30.
Article28. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003; 52:2795–804.
Article29. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295:1681–7.
Article30. Park SE, Lee BW, Kim JH, Lee WJ, Cho JH, Jung CH, et al. Effect of gemigliptin on glycaemic variability in patients with type 2 diabetes (STABLE study). Diabetes Obes Metab. 2017; 19:892–6.
Article31. Kim NH, Kim DL, Kim KJ, Kim NH, Choi KM, Baik SH, et al. Effects of vildagliptin or pioglitazone on glycemic variability and oxidative stress in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a 16-week, randomised, open label, pilot study. Endocrinol Metab (Seoul). 2017; 32:241–7.
Article32. Kim HS, Shin JA, Lee SH, Kim ES, Cho JH, Son HY, et al. A comparative study of the effects of a dipeptidyl peptidase-IV inhibitor and sulfonylurea on glucose variability in patients with type 2 diabetes with inadequate glycemic control on metformin. Diabetes Technol Ther. 2013; 15:810–6.
Article33. FLAT-SUGAR Trial Investigators. Glucose variability in a 26-week randomized comparison of mealtime treatment with rapid-acting insulin versus GLP-1 agonist in participants with type 2 diabetes at high cardiovascular risk. Diabetes Care. 2016; 39:973–81.34. Al-Jobori H, Daniele G, Cersosimo E, Triplitt C, Mehta R, Norton L, et al. Empagliflozin and kinetics of renal glucose transport in healthy individuals and individuals with type 2 diabetes. Diabetes. 2017; 66:1999–2006.
Article35. Rave K, Nosek L, Posner J, Heise T, Roggen K, van Hoogdalem EJ. Renal glucose excretion as a function of blood glucose concentration in subjects with type 2 diabetes: results of a hyperglycaemic glucose clamp study. Nephrol Dial Transplant. 2006; 21:2166–71.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pioglitazone as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Efficacy of Gemigliptin Add-on to Dapagliflozin and Metformin in Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo-Controlled Study (SOLUTION)
- Efficacy and Safety of Enavogliflozin versus Dapagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A 24-Week, Double-Blind, Randomized Trial
- Glycemic Variability and Diabetes Mellitus
- Efficacy and Safety of Alogliptin-Pioglitazone Combination for Type 2 Diabetes Mellitus Poorly Controlled with Metformin: A Multicenter, Double-Blind Randomized Trial