Diabetes Metab J.
2024 Sep;48(5):915-928. 10.4093/dmj.2023.0259.
Efficacy and Safety of Alogliptin-Pioglitazone Combination for Type 2 Diabetes Mellitus Poorly Controlled with Metformin: A Multicenter, Double-Blind Randomized Trial
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2MedicalExcellence Inc., Seoul, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea
- 4Division of Endocrinology and Metabolism, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, Korea
- 5Division of Endocrinology and Metabolism, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 6Division of Endocrinology and Metabolism, Bundang Jesaeng Hospital, Seongnam, Korea
- 7Division of Endocrinology and Metabolism, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 8Department of Endocrinology, Korea University Guro Hospital, Seoul, Korea
- 9Department of Medical Informatics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2559001
- DOI: http://doi.org/10.4093/dmj.2023.0259
Abstract
- Background
Guidelines for switching to triple combination therapy directly after monotherapy failure are limited. This study investigated the efficacy, long-term sustainability, and safety of either mono or dual add-on therapy using alogliptin and pioglitazone for patients with type 2 diabetes mellitus (T2DM) who did not achieve their target glycemic range with metformin monotherapy.
Methods
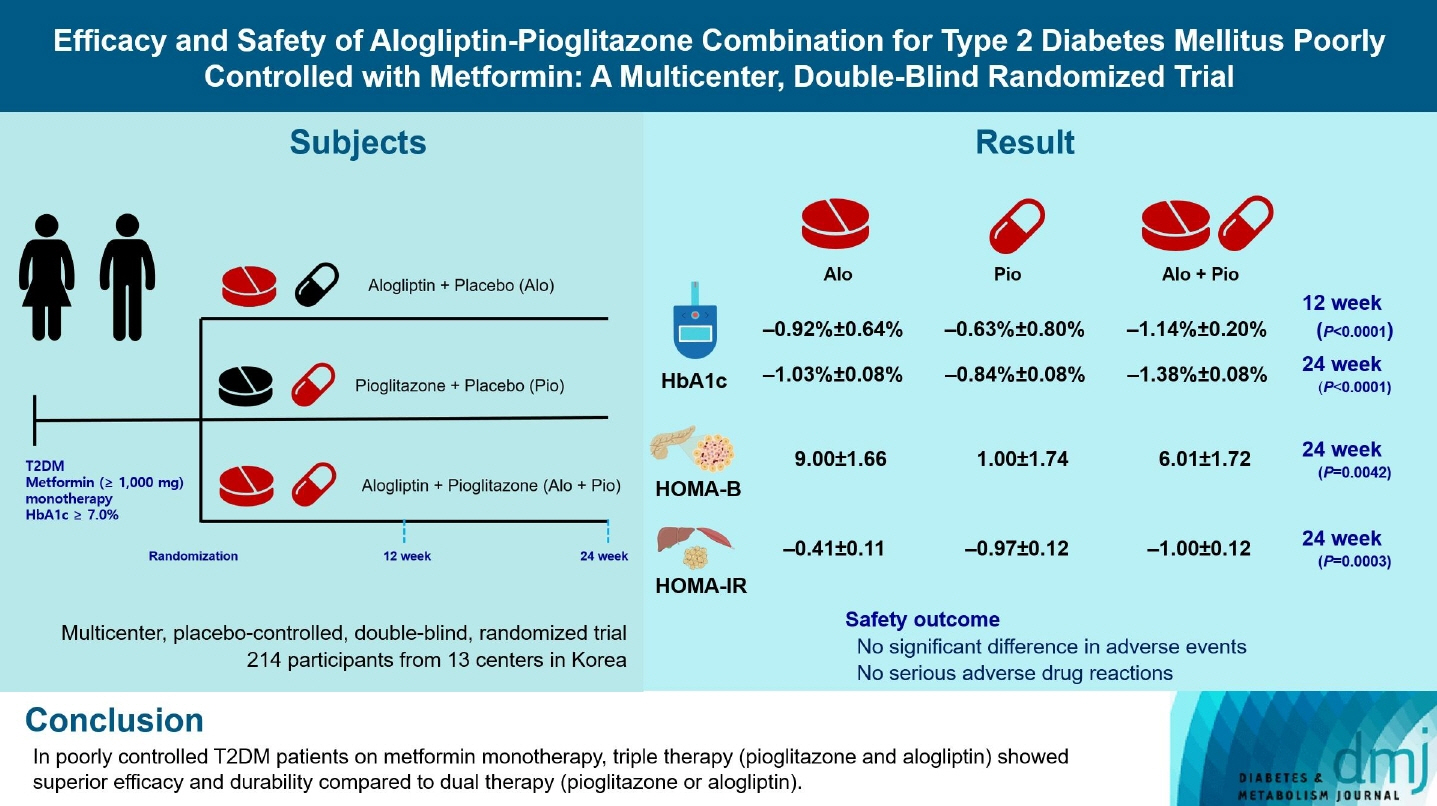
The Practical Evidence of Antidiabetic Combination Therapy in Korea (PEAK) was a multicenter, placebo-controlled, double-blind, randomized trial. A total of 214 participants were randomized to receive alogliptin+pioglitazone (Alo+Pio group, n=70), alogliptin (Alo group, n=75), or pioglitazone (Pio group, n=69). The primary outcome was the difference in glycosylated hemoglobin (HbA1c) levels between the three groups at baseline to 24 weeks. For durability, the achievement of HbA1c levels <7% and <6.5% was compared in each group. The number of adverse events was investigated for safety.
Results
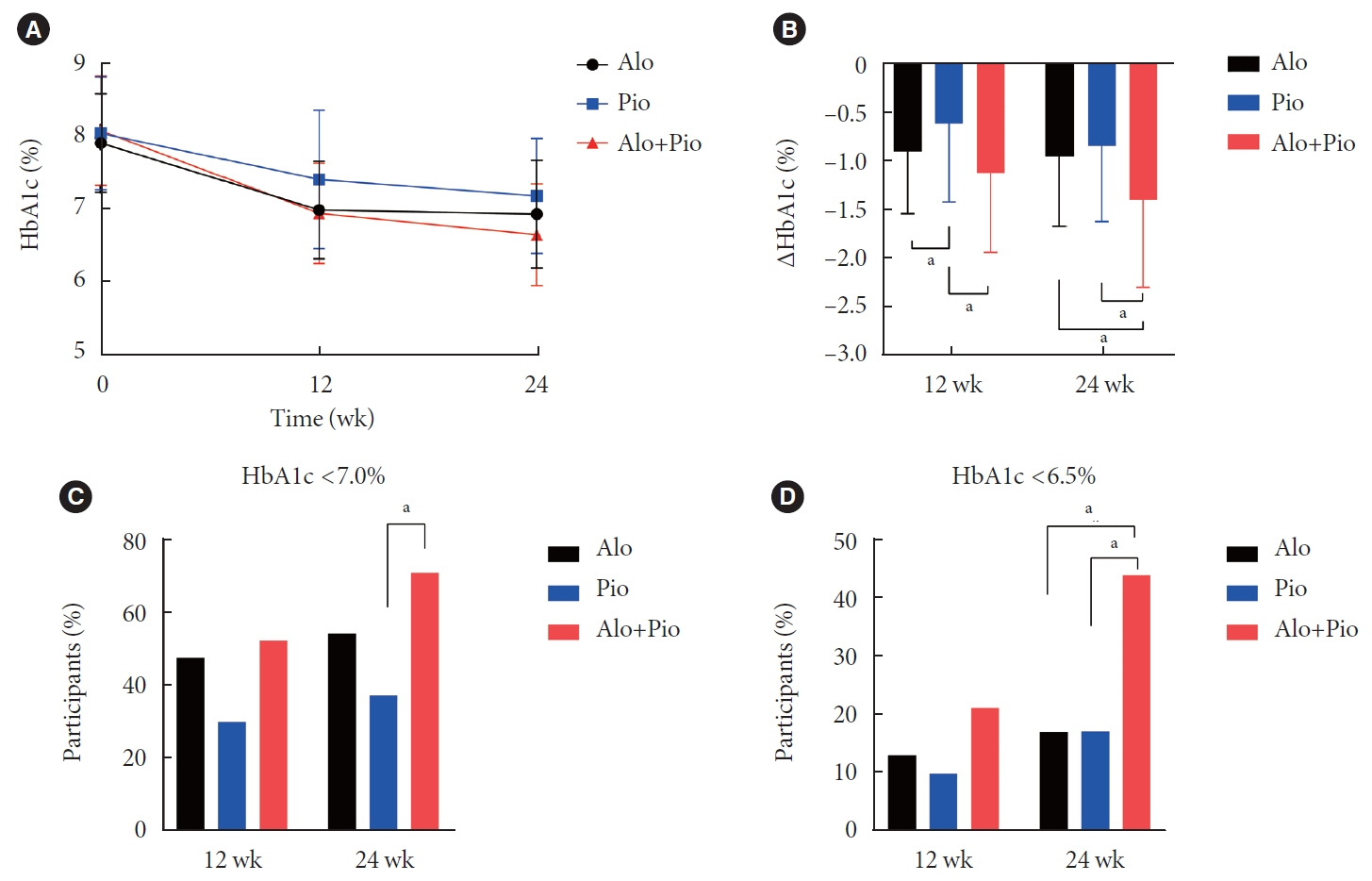
After 24 weeks of treatment, the change of HbA1c in the Alo+Pio, Alo, and Pio groups were –1.38%±0.08%, –1.03%±0.08%, and –0.84%±0.08%, respectively. The Alo+Pio group had significantly lower HbA1c levels than the other groups (P=0.0063, P<0.0001) and had a higher proportion of patients with target HbA1c achievement. In addition, insulin sensitivity and β-cell function, lipid profiles, and other metabolic indicators were also improved. There were no significant safety issues in patients treated with triple combination therapy.
Conclusion
Early combination triple therapy showed better efficacy and durability than the single add-on (dual) therapy. Therefore, combination therapy with metformin, alogliptin, and pioglitazone is a valuable early treatment option for T2DM poorly controlled with metformin monotherapy.
Keyword
Figure
Reference
-
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019; 157:107843.
Article2. Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J. 2022; 46:417–26.
Article3. Matthews DR, Paldanius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019; 394:1519–29.
Article4. Abdul-Ghani MA, Puckett C, Triplitt C, Maggs D, Adams J, Cersosimo E, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes: results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab. 2015; 17:268–75.5. Yoo SJ, Chang SA, Sohn TS, Kwon HS, Lee JM, Moon S, et al. Long-term glycaemic durability of early combination therapy strategy versus metformin monotherapy in Korean patients with newly diagnosed type 2 diabetes mellitus. Diabetes Metab J. 2021; 45:954–9.
Article6. Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021; 45:461–81.
Article7. Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012; 23:205–15.
Article8. Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020; 16:642–53.
Article9. Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009; 32:187–202.
Article10. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016; 165:305–15.
Article11. Covington P, Christopher R, Davenport M, Fleck P, Mekki QA, Wann ER, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther. 2008; 30:499–512.
Article12. Feng J, Zhang Z, Wallace MB, Stafford JA, Kaldor SW, Kassel DB, et al. Discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. J Med Chem. 2007; 50:2297–300.
Article13. Ueki K, Tanizawa Y, Nakamura J, Yamada Y, Inagaki N, Watada H, et al. Long-term safety and efficacy of alogliptin, a DPP-4 inhibitor, in patients with type 2 diabetes: a 3-year prospective, controlled, observational study (J-BRAND Registry). BMJ Open Diabetes Res Care. 2021; 9:e001787.
Article14. DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Efficacy and tolerability of the DPP-4 inhibitor alogliptin combined with pioglitazone, in metformin-treated patients with type 2 diabetes. J Clin Endocrinol Metab. 2012; 97:1615–22.
Article15. Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther. 2012; 29:14–25.
Article16. Betteridge DJ. Effects of pioglitazone on lipid and lipoprotein metabolism. Diabetes Obes Metab. 2007; 9:640–7.
Article17. Nagashima K, Lopez C, Donovan D, Ngai C, Fontanez N, Bensadoun A, et al. Effects of the PPARgamma agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J Clin Invest. 2005; 115:1323–32.18. Chan JC, Paldanius PM, Mathieu C, Stumvoll M, Matthews DR, Del Prato S. Early combination therapy delayed treatment escalation in newly diagnosed young-onset type 2 diabetes: a subanalysis of the VERIFY study. Diabetes Obes Metab. 2021; 23:245–51.19. Ji L, Chan JC, Yu M, Yoon KH, Kim SG, Choi SH, et al. Early combination versus initial metformin monotherapy in the management of newly diagnosed type 2 diabetes: an East Asian perspective. Diabetes Obes Metab. 2021; 23:3–17.20. Ahren B, Foley JE. Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia. 2016; 59:907–17.
Article21. Gastaldelli A, Casolaro A, Pettiti M, Nannipieri M, Ciociaro D, Frascerra S, et al. Effect of pioglitazone on the metabolic and hormonal response to a mixed meal in type II diabetes. Clin Pharmacol Ther. 2007; 81:205–12.
Article22. Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005; 28:1547–54.
Article23. Zhang LH, Kamanna VS, Ganji SH, Xiong XM, Kashyap ML. Pioglitazone increases apolipoprotein A-I production by directly enhancing PPRE-dependent transcription in HepG2 cells. J Lipid Res. 2010; 51:2211–22.
Article24. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009; 301:2129–40.25. Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006; 368:1681–8.
Article26. Kim JM, Kim SS, Kim JH, Kim MK, Kim TN, Lee SH, et al. Efficacy and safety of pioglitazone versus glimepiride after metformin and alogliptin combination therapy: a randomized, open-label, multicenter, parallel-controlled study. Diabetes Metab J. 2020; 44:67–77.
Article27. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, MassiBenedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet. 2005; 366:1279–89.28. Yaghi S, Furie KL, Viscoli CM, Kamel H, Gorman M, Dearborn J, et al. Pioglitazone prevents stroke in patients with a recent transient ischemic attack or ischemic stroke: a planned secondary analysis of the IRIS trial (Insulin Resistance Intervention After Stroke). Circulation. 2018; 137:455–63.
Article29. Young LH, Viscoli CM, Curtis JP, Inzucchi SE, Schwartz GG, Lovejoy AM, et al. Cardiac outcomes after ischemic stroke or transient ischemic attack: effects of pioglitazone in patients with insulin resistance without diabetes mellitus. Circulation. 2017; 135:1882–93.
Article30. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016; 374:1321–31.
Article31. Tu WJ, Zhao Z, Yin P, Cao L, Zeng J, Chen H, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. 2023; 6:e231455.
Article32. Venketasubramanian N, Yoon BW, Pandian J, Navarro JC. Stroke epidemiology in South, East, and South-East Asia: a review. J Stroke. 2017; 19:286–94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Pioglitazone versus Glimepiride after Metformin and Alogliptin Combination Therapy: A Randomized, Open-Label, Multicenter, Parallel-Controlled Study
- Comparison of Efficacy of Glimepiride, Alogliptin, and Alogliptin-Pioglitazone as the Initial Periods of Therapy in Patients with Poorly Controlled Type 2 Diabetes Mellitus: An Open-Label, Multicenter, Randomized, Controlled Study
- Letter: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2019;43;276-86)
- Pioglitazone as Add-on Therapy in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Dapagliflozin and Metformin: Double-Blind, Randomized, Placebo-Controlled Trial
- Response: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes metab J 2019;43;276-86)