J Korean Assoc Oral Maxillofac Surg.
2018 Dec;44(6):259-268. 10.5125/jkaoms.2018.44.6.259.
Combined effect of recombinant human bone morphogenetic protein-2 and low level laser irradiation on bisphosphonate-treated osteoblasts
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Pusan National University, Yangsan, Korea. ssh8080@pusan.ac.kr
- 2Department of Oral Anatomy, School of Dentistry, Pusan National University, Yangsan, Korea.
- 3Department of Oral and Maxillofacial Surgery, Dong-A University Hospital, Busan, Korea.
- KMID: 2467172
- DOI: http://doi.org/10.5125/jkaoms.2018.44.6.259
Abstract
OBJECTIVES
The purpose of this study was to evaluate the synergic effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) and low-level laser therapy (LLLT) on bisphosphonate-treated osteoblasts.
MATERIALS AND METHODS
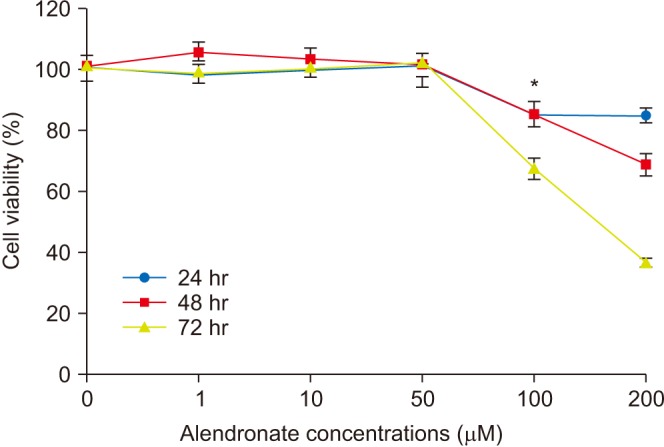
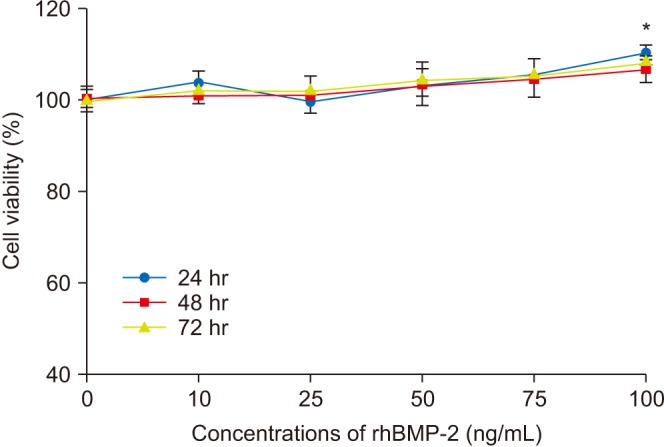
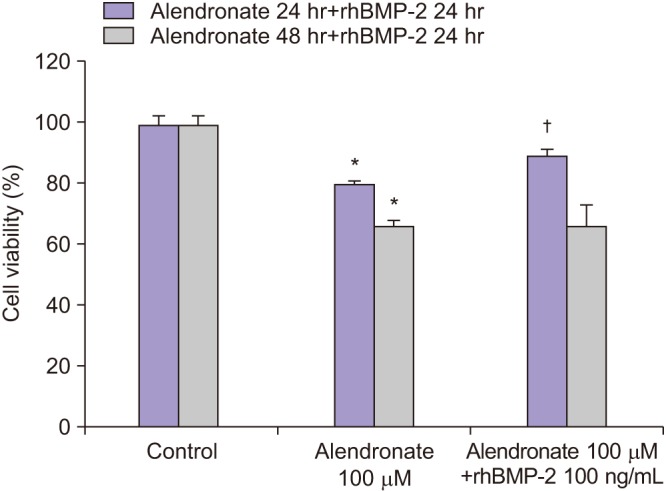
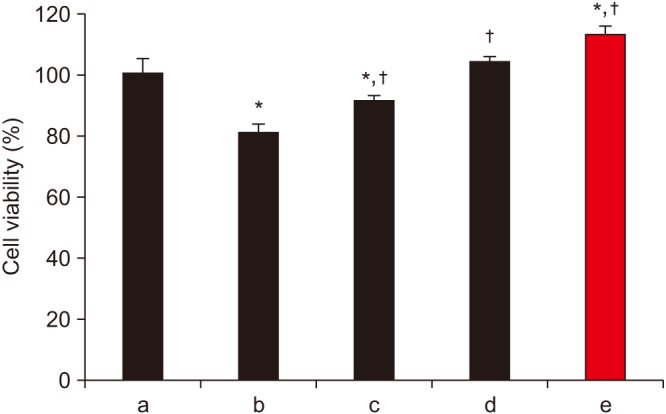
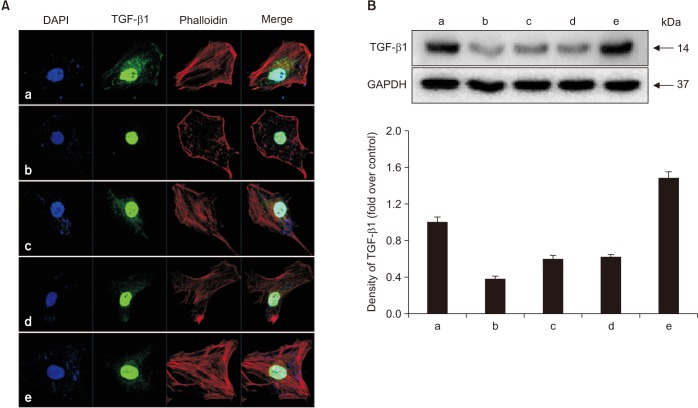
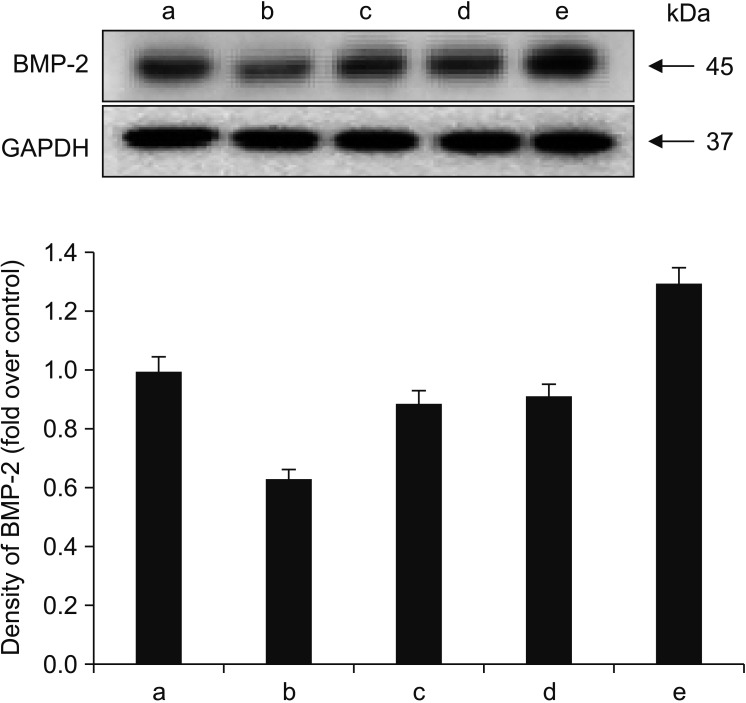
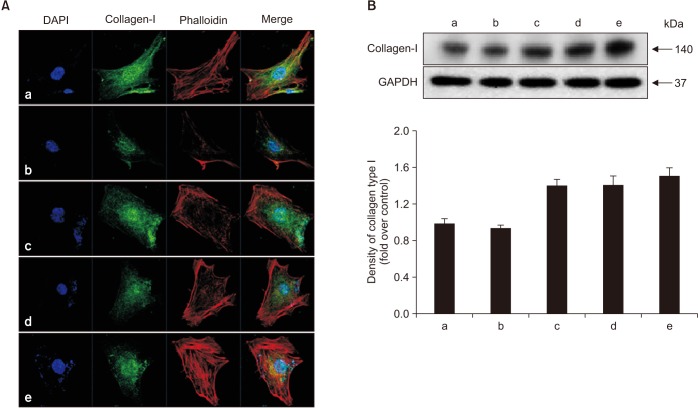
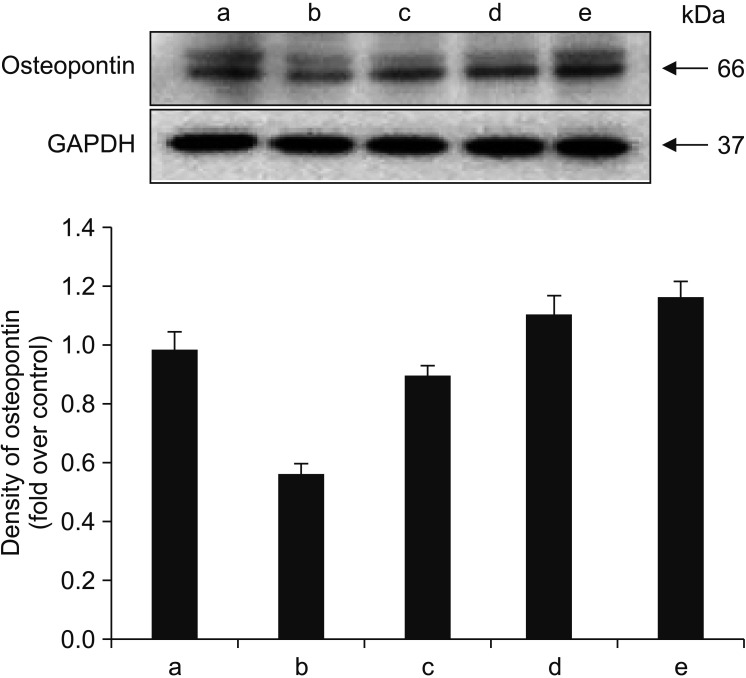
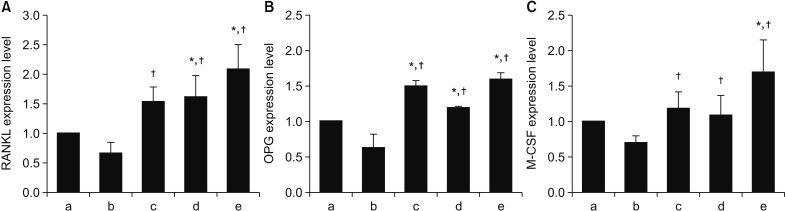
Human fetal osteoblast cells (hFOB 1.19) were cultured with 100 µM alendronate. Low-level Ga-Al-As laser alone or with 100 ng/mL rhBMP-2 was then applied. Cell viability was measured with MTT assay. The expression levels of receptor activator of nuclear factor kappa-B ligand (RANKL), macrophage colony-stimulating factor (M-CSF), and osteoprotegerin (OPG) were analyzed for osteoblastic activity inducing osteoclastic activity. Collagen type and transforming growth factor beta-1 were also evaluated for bone matrix formation.
RESULTS
The results showed that rhBMP-2 and LLLT had a synergic effect on alendronate-treated osteoblasts for enhancing osteoblastic activity and bone matrix formation. Between rhBMP-2 and LLLT, rhBMP-2 exhibited a greater effect, but did not show a significant difference.
CONCLUSION
rhBMP-2 and LLLT have synergic effects on bisphosphonate-treated osteoblasts through enhancement of osteoblastic activity and bone formation activity.
MeSH Terms
-
Alendronate
Bone Matrix
Bone Morphogenetic Protein 2
Cell Survival
Collagen
Humans*
Low-Level Light Therapy
Macrophage Colony-Stimulating Factor
Osteoblasts*
Osteoclasts
Osteogenesis
Osteoprotegerin
Transforming Growth Factors
Alendronate
Bone Morphogenetic Protein 2
Collagen
Macrophage Colony-Stimulating Factor
Osteoprotegerin
Transforming Growth Factors
Figure
Reference
-
1. Boonyapakorn T, Schirmer I, Reichart PA, Sturm I, Massenkeil G. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol. 2008; 44:857–869. PMID: 18282788.
Article2. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003; 61:1115–1117. PMID: 12966493.
Article3. Lee JK, Kim KW, Choi JY, Moon SY, Kim SG, Kim CH, et al. Bisphosphonates-related osteonecrosis of the jaw in Korea: a preliminary report. J Korean Assoc Oral Maxillofac Surg. 2013; 39:9–13. PMID: 24471011.
Article4. Woo SB, Hellstein JW, Kalmar JP. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006; 144:753–761. PMID: 16702591.5. Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007; 65:415–423. PMID: 17307586.
Article6. Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996; 97:2692–2696. PMID: 8675678.
Article7. Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest. 1993; 91:2004–2011. PMID: 8486770.
Article8. Murakami H, Takahashi N, Sasaki T, Udagawa N, Tanaka S, Nakamura I, et al. A possible mechanism of the specific action of bisphosphonates on osteoclasts: tiludronate preferentially affects polarized osteoclasts having ruffled borders. Bone. 1995; 17:137–144. PMID: 8554921.
Article9. Aubin JE, Triffitt JT. Mesenchymal stem cells and the osteoblast lineage. In : Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. 2nd ed. New York: Academic;2002.10. Tay JY, Bay BH, Yeo JF, Harris M, Meghji S, Dheen ST. Identification of RANKL in osteolytic lesions of the facial skeleton. J Dent Res. 2004; 83:349–353. PMID: 15044512.
Article11. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998; 93:165–176. PMID: 9568710.
Article12. Khadra M, Lyngstadaas SP, Haanaes HR, Mustafa K. Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials. 2005; 26:3503–3509. PMID: 15621240.
Article14. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004; 22:233–241. PMID: 15621726.
Article15. Kwon TK, Song JM, Kim IR, Park BS, Kim CH, Cheong IK, et al. Effect of recombinant human bone morphogenetic protein-2 on bisphosphonate-treated osteoblasts. J Korean Assoc Oral Maxillofac Surg. 2014; 40:291–296. PMID: 25551094.
Article16. Marx RE. Clinical concerns of alendronate use. J Oral Maxillofac Surg. 2008; 66:1322. PMID: 18486814.
Article17. Rustemeyer J, Bremerich A. Bisphosphonate-associated osteonecrosis of the jaw: what do we currently know? A survey of knowledge given in the recent literature. Clin Oral Investig. 2009; 14:59–64.
Article18. García-Moreno C, Serrano S, Nacher M, Farré M, Díez A, Mariñoso ML, et al. Effect of alendronate on cultured normal human osteoblasts. Bone. 1998; 22:233–239. PMID: 9580147.
Article19. Vitté C, Fleisch H, Guenther HL. Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast-mediated resorption. Endocrinology. 1996; 137:2324–2333. PMID: 8641182.
Article20. Nishikawa M, Akatsu T, Katayama Y, Yasutomo Y, Kado S, Kugal N, et al. Bisphosphonates act on osteoblastic cells and inhibit osteoclast formation in mouse marrow cultures. Bone. 1996; 18:9–14. PMID: 8717530.
Article21. Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J bone Miner Res. 2008; 23:715–720. PMID: 18433304.
Article22. Choi ST, Kim JH, Kang EJ, Lee SW, Park MC, Park YB, et al. Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology (Oxford). 2008; 47:1775–1779. PMID: 18854347.
Article23. Ashizawa N, Graf K, Do YS, Nunohiro T, Giachelli CM, Meehan WP, et al. Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. J Clin Invest. 1996; 98:2218–2227. PMID: 8941637.
Article24. Murry CE, Giachelli CM, Schwartz SM, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol. 1994; 145:1450–1462. PMID: 7992848.25. Ikeda T, Shirasawa T, Esaki Y, Yoshiki S, Hirokawa K. Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta. J Clin Invest. 1993; 92:2814–2820. PMID: 8254036.
Article26. Reinholt FP, Hultenby K, Oldberg A, Heinegård D. Osteopontin: a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990; 87:4473–4475. PMID: 1693772.27. Itoh K, Udagawa N, Katagiri T, Iemura S, Ueno N, Yasuda H, et al. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factorkappaB ligand. Endocrinology. 2001; 142:3656–3662. PMID: 11459815.28. Zheng Y, Wang L, Zhang X, Zhang X, Gu Z, Wu G. BMP2/7 heterodimer can modulate all cellular events of the in vitro RANKL-mediated osteoclastogenesis, respectively, in different dose patterns. Tissue Eng Part A. 2012; 18:621–630. PMID: 21981321.29. Goldman L, Goldman B, Van Lieu N. Current laser dentistry. Lasers Surg Med. 1987; 6:559–562. PMID: 3573930.
Article30. Stein A, Benayahu D, Maltz L, Oron U. Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg. 2005; 23:161–166. PMID: 15910179.31. Renno AC, McDonnell PA, Parizotto NA, Laakso EL. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg. 2007; 25:275–280. PMID: 17803384.
Article32. Kiyosaki T, Mitsui N, Suzuki N, Shimizu N. Low-level laser therapy stimulates mineralization via increased Runx2 expression and ERK phosphorylation in osteoblasts. Photomed Laser Surg. 2010; 28(Suppl 1):S167–S172. PMID: 20649430.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined effect of bisphosphonate and recombinant human bone morphogenetic protein 2 on bone healing of rat calvarial defects
- Effect of recombinant human bone morphogenetic protein-2 on bisphosphonate-treated osteoblasts
- Effect of low-level laser therapy on bisphosphonate-treated osteoblasts
- Spinal Fusion Based on Ex Vivo Gene Therapy Using Recombinant Human BMP Adenoviruses
- Sinus floor augmentation using a mineralized freeze-dried bone graft combined with recombinant human bone morphogenetic protein-2: clinical and histological results of three cases