Korean J Physiol Pharmacol.
2020 Jan;24(1):11-18. 10.4196/kjpp.2020.24.1.11.
Neuroprotective potential of imatinib in global ischemia-reperfusion-induced cerebral injury: possible role of Janus-activated kinase 2/signal transducer and activator of transcription 3 and connexin 43
- Affiliations
-
- 1Department of Pediatrics, Shaanxi Provincial People's Hospital, The Affiliated Hospital of Xi'an Medical University, The Third Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710068, Shaanxi, China. sxxaped@sina.com
- 2Central Laboratory, Shaanxi Provincial People's Hospital, The Affiliated Hospital of Xi'an Medical University, The Third Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710068, Shaanxi, China.
- 3Department of Neurology, Xijing Hospital, Air Force Medical University, Xi'an 710032, Shaanxi, China.
- KMID: 2466565
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.11
Abstract
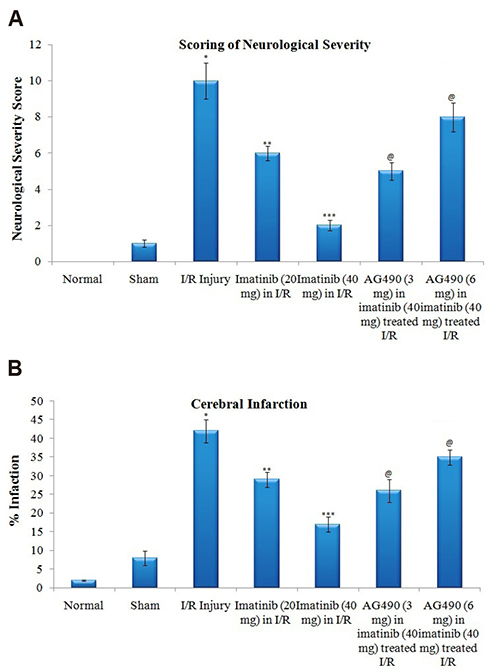
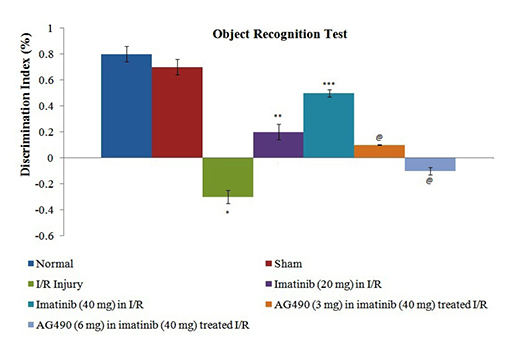
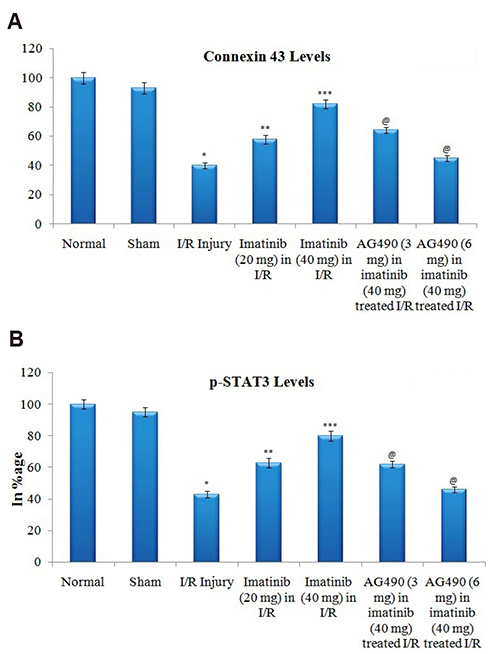
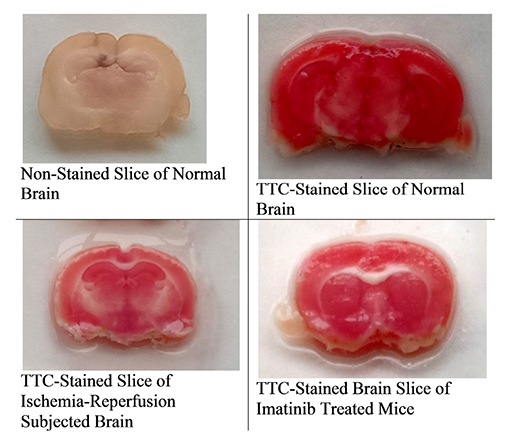
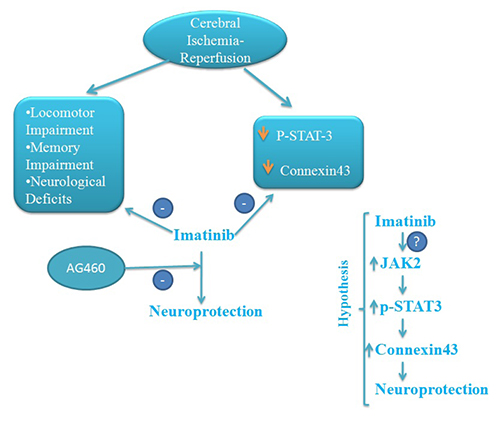
- The present study was aimed to explore the neuroprotective role of imatinib in global ischemia-reperfusion-induced cerebral injury along with possible mechanisms. Global ischemia was induced in mice by bilateral carotid artery occlusion for 20 min, which was followed by reperfusion for 24 h by restoring the blood flow to the brain. The extent of cerebral injury was assessed after 24 h of global ischemia by measuring the locomotor activity (actophotometer test), motor coordination (inclined beam walking test), neurological severity score, learning and memory (object recognition test) and cerebral infarction (triphenyl tetrazolium chloride stain). Ischemia-reperfusion injury produced significant cerebral infarction, impaired the behavioral parameters and decreased the expression of connexin 43 and phosphorylated signal transducer and activator of transcription 3 (p-STAT3) in the brain. A single dose administration of imatinib (20 and 40 mg/kg) attenuated ischemia-reperfusion-induced behavioral deficits and the extent of cerebral infarction along with the restoration of connexin 43 and p-STAT3 levels. However, administration of AG490, a selective Janus-activated kinase 2 (JAK2)/STAT3 inhibitor, abolished the neuroprotective actions of imatinib and decreased the expression of connexin 43 and p-STAT3. It is concluded that imatinib has the potential of attenuating global ischemia-reperfusion-induced cerebral injury, which may be possibly attributed to activation of JAK2/STAT3 signaling pathway along with the increase in the expression of connexin 43.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Dexmedetomidine alleviates blood-brain barrier disruption in rats after cerebral ischemia-reperfusion by suppressing JNK and p38 MAPK signaling
Canmin Zhu, Dili Wang, Chang Chang, Aofei Liu, Ji Zhou, Ting Yang, Yuanfeng Jiang, Xia Li, Weijian Jiang
Korean J Physiol Pharmacol. 2024;28(3):239-252. doi: 10.4196/kjpp.2024.28.3.239.
Reference
-
1. Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, Apperley J, Cervantes F, Cortes J, Deininger M, Gratwohl A, Guilhot F, Horowitz M, Hughes T, Kantarjian H, Larson R, Niederwieser D, Silver R, Hehlmann R. European LeukemiaNet. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006; 108:1809–1820.
Article2. Ben Ami E, Demetri GD. A safety evaluation of imatinib mesylate in the treatment of gastrointestinal stromal tumor. Expert Opin Drug Saf. 2016; 15:571–578.
Article3. Kumar M, Kulshrestha R, Singh N, Jaggi AS. Expanding spectrum of anticancer drug, imatinib, in the disorders affecting brain and spinal cord. Pharmacol Res. 2019; 143:86–96.
Article4. Wu R, Chen H, Ma J, He Q, Huang Q, Liu Q, Li M, Yuan Z. c-Abl-p38α signaling plays an important role in MPTP-induced neuronal death. Cell Death Differ. 2016; 23:542–552.
Article5. Cancino GI, Perez de, Castro PU, Toledo EM, von Bernhardi R, Alvarez AR. c-Abl tyrosine kinase modulates tau pathology and Cdk5 phosphorylation in AD transgenic mice. Neurobiol Aging. 2011; 32:1249–1261.
Article6. Merali Z, Leung J, Mikulis D, Silver F, Kassner A. Longitudinal assessment of imatinib's effect on the blood-brain barrier after ischemia/reperfusion injury with permeability MRI. Transl Stroke Res. 2015; 6:39–49.
Article7. Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008; 14:731–737.
Article8. Su EJ, Fredriksson L, Kanzawa M, Moore S, Folestad E, Stevenson TK, Nilsson I, Sashindranath M, Schielke GP, Warnock M, Ragsdale M, Mann K, Lawrence AL, Medcalf RL, Eriksson U, Murphy GG, Lawrence DA. Imatinib treatment reduces brain injury in a murine model of traumatic brain injury. Front Cell Neurosci. 2015; 9:385.
Article9. Zhan Y, Krafft PR, Lekic T, Ma Q, Souvenir R, Zhang JH, Tang J. Imatinib preserves blood-brain barrier integrity following experimental subarachnoid hemorrhage in rats. J Neurosci Res. 2015; 93:94–103.
Article10. Ma Q, Huang B, Khatibi N, Rolland W 2nd, Suzuki H, Zhang JH, Tang J. PDGFR-α inhibition preserves blood-brain barrier after intracerebral hemorrhage. Ann Neurol. 2011; 70:920–931.
Article11. Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006; 24:1–21.
Article12. Wiklund L, Martijn C, Miclescu A, Semenas E, Rubertsson S, Sharma HS. Central nervous tissue damage after hypoxia and reperfusion in conjunction with cardiac arrest and cardiopulmonary resuscitation: mechanisms of action and possibilities for mitigation. Int Rev Neurobiol. 2012; 102:173–187.13. Tang Y, Tong X, Li Y, Jiang G, Yu M, Chen Y, Dong S. JAK2/STAT3 pathway is involved in the protective effects of epidermal growth factor receptor activation against cerebral ischemia/reperfusion injury in rats. Neurosci Lett. 2018; 662:219–226.
Article14. Li Y, Zhang X, Cui L, Chen R, Zhang Y, Zhang C, Zhu X, He T, Shen Z, Dong L, Zhao J, Wen Y, Zheng X, Li P. Salvianolic acids enhance cerebral angiogenesis and neurological recovery by activating JAK2/ STAT3 signaling pathway after ischemic stroke in mice. J Neurochem. 2017; 143:87–99.15. Chen M, Zou W, Chen M, Cao L, Ding J, Xiao W, Hu G. Ginkgolide K promotes angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 pathway. Eur J Pharmacol. 2018; 833:221–229.
Article16. Xing L, Yang T, Cui S, Chen G. Connexin hemichannels in astrocytes: role in CNS disorders. Front Mol Neurosci. 2019; 12:23.
Article17. Liu WJ, Yang J. Preferentially regulated expression of connexin 43 in the developing spiral ganglion neurons and afferent terminals in post-natal rat cochlea. Eur J Histochem. 2015; 59:2464.
Article18. Wu LY, Yu XL, Feng LY. Connexin 43 stabilizes astrocytes in a stroke-like milieu to facilitate neuronal recovery. Acta Pharmacol Sin. 2015; 36:928–938.
Article19. Schulz R, Görge PM, Görbe A, Ferdinandy P, Lampe PD, Leybaert L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther. 2015; 153:90–106.
Article20. Yi C, Koulakoff A, Giaume C. Astroglial connexins as a therapeutic target for Alzheimer's disease. Curr Pharm Des. 2017; 23:4958–4968.
Article21. Weintraub MK, Bisson CM, Nouri JN, Vinson BT, Eimerbrink MJ, Kranjac D, Boehm GW, Chumley MJ. Imatinib methanesulfonate reduces hippocampal amyloid-β and restores cognitive function following repeated endotoxin exposure. Brain Behav Immun. 2013; 33:24–28.22. Cancino GI, Toledo EM, Leal NR, Hernandez DE, Yévenes LF, Inestrosa NC, Alvarez AR. STI571 prevents apoptosis, tau phosphorylation and behavioural impairments induced by Alzheimer's beta-amyloid deposits. Brain. 2008; 131(Pt 9):2425–2442.23. Chu J, Lauretti E, Craige CP, Praticò D. Pharmacological modulation of GSAP reduces amyloid-β levels and tau phosphorylation in a mouse model of Alzheimer's disease with plaques and tangles. J Alzheimers Dis. 2014; 41:729–737.
Article24. Chen B, Yang L, Chen J, Chen Y, Zhang L, Wang L, Li X, Li Y, Yu H. Inhibition of Connexin43 hemichannels with Gap19 protects cerebral ischemia/reperfusion injury via the JAK2/STAT3 pathway in mice. Brain Res Bull. 2019; 146:124–135.
Article25. Kim SJ, Lee SR. Protective effect of melatonin against transient global cerebral ischemia-induced neuronal cell damage via inhibition of matrix metalloproteinase-9. Life Sci. 2014; 94:8–16.
Article26. He JT, Li H, Yang L, Cheng KL. Involvement of endothelin-1, H2S and Nrf2 in beneficial effects of remote ischemic preconditioning in global cerebral ischemia-induced vascular dementia in mice. Cell Mol Neurobiol. 2019; 39:671–686.
Article27. Rodriguez R, Santiago-Mejia J, Gomez C, San-Juan ER. A simplified procedure for the quantitative measurement of neurological deficits after forebrain ischemia in mice. J Neurosci Methods. 2005; 147:22–28.
Article28. Ya BL, Li HF, Wang HY, Wu F, Xin Q, Cheng HJ, Li WJ, Lin N, Ba ZH, Zhang RJ, Liu Q, Li YN, Bai B, Ge F. 5-HMF attenuates striatum oxidative damage via Nrf2/ARE signaling pathway following transient global cerebral ischemia. Cell Stress Chaperones. 2017; 22:55–65.
Article29. Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T. Object recognition test in mice. Nat Protoc. 2013; 8:2531–2537.
Article30. Yang Y, Lu F, Zhuang L, Yang S, Kong Y, Tan W, Gong Z, Zhan S. Combined preconditioning with hypoxia and GYKI-52466 protects rats from cerebral ischemic injury by HIF-1α/eNOS pathway. Am J Transl Res. 2017; 9:5308–5319.31. Okuno S, Nakase H, Sakaki T. Comparative study of 2,3,5-triphenyltetrazolium chloride (TTC) and hematoxylin-eosin staining for quantification of early brain ischemic injury in cats. Neurol Res. 2001; 23:657–661.
Article32. Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009; 30:201–211.
Article33. Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci U S A. 2001; 98:13391–13395.
Article34. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012; 13:93–110.
Article35. Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017; (126):55718.
Article36. Estrada LD, Chamorro D, Yañez MJ, Gonzalez M, Leal N, von Bernhardi R, Dulcey AE, Marugan J, Ferrer M, Soto C, Zanlungo S, Inestrosa NC, Alvarez AR. Reduction of blood amyloid-β oligomers in Alzheimer's disease transgenic mice by c-Abl kinase inhibition. J Alzheimers Dis. 2016; 54:1193–1205.
Article37. Zhao Y, Xue Y, Liu Z, Ren S, Guan X, Li M, Zhao X, Song Y, Ren X. Role of the Janus kinase 2/signal transducers and activators of transcription 3 pathway in the protective effect of remote ischemia preconditioning against cerebral ischemia-reperfusion injury in rats. Neuroreport. 2019; 30:664–670.
Article38. Jung JE, Kim GS, Chan PH. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke. 2011; 42:3574–3579.
Article39. Tu S, Xiao F, Min X, Chen H, Fan X, Cao K. Catechin attenuates coronary heart disease in a rat model by inhibiting inflammation. Cardiovasc Toxicol. 2018; 18:393–399.
Article40. Zhu Y, Liu Z, Peng YP, Qiu YH. Interleukin-10 inhibits neuroin-flammation-mediated apoptosis of ventral mesencephalic neurons via JAK-STAT3 pathway. Int Immunopharmacol. 2017; 50:353–360.
Article41. Sharma S, Yang B, Xi X, Grotta JC, Aronowski J, Savitz SI. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 2011; 1373:189–194.
Article42. Vicario N, Zappalà A, Calabrese G, Gulino R, Parenti C, Gulisano M, Parenti R. Connexins in the central nervous system: physiological traits and neuroprotective targets. Front Physiol. 2017; 8:1060.
Article43. Hanstein R, Trotter J, Behl C, Clement AB. Increased connexin 43 expression as a potential mediator of the neuroprotective activity of the corticotropin-releasing hormone. Mol Endocrinol. 2009; 23:1479–1493.
Article44. Chen YS, Green CR, Teague R, Perrett J, Danesh-Meyer HV, Toth I, Rupenthal ID. Intravitreal injection of lipoamino acid-modified connexin43 mimetic peptide enhances neuroprotection after retinal ischemia. Drug Deliv Transl Res. 2015; 5:480–488.
Article45. Ma Y, Bu J, Dang H, Sha J, Jing Y, Shan-jiang AI, Li H, Zhu Y. Inhibition of adenosine monophosphate-activated protein kinase reduces glial cell-mediated inflammation and induces the expression of Cx43 in astroglias after cerebral ischemia. Brain Res. 2015; 1605:1–11.
Article46. Kim IS, Ganesan P, Choi DK. Cx43 mediates resistance against MPP+-induced apoptosis in SH-SY5Y neuroblastoma cells via modulating the mitochondrial apoptosis pathway. Int J Mol Sci. 2016; 17:E1819.
Article47. Nakase T, Söhl G, Theis M, Willecke K, Naus CC. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am J Pathol. 2004; 164:2067–2075.
Article48. Ozog MA, Bernier SM, Bates DC, Chatterjee B, Lo CW, Naus CC. The complex of ciliary neurotrophic factor-ciliary neurotrophic factor receptor alpha up-regulates connexin43 and intercellular coupling in astrocytes via the Janus tyrosine kinase/signal transducer and activator of transcription pathway. Mol Biol Cell. 2004; 15:4761–4774.49. Gao S, Li S, Duan X, Gu Z, Ma Z, Yuan X, Feng X, Wang H. Inhibition of glycogen synthase kinase 3 beta (GSK3β) suppresses the progression of esophageal squamous cell carcinoma by modifying STAT3 activity. Mol Carcinog. 2017; 56:2301–2316.
Article50. Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. J Biol Chem. 2008; 283:21934–21944.
Article51. Reddiconto G, Toto C, Palamà I, De Leo S, de Luca E, De Matteis S, Dini L, Passerini CG, Di Renzo N, Maffia M, Coluccia AM. Targeting of GSK3β promotes imatinib-mediated apoptosis in quiescent CD34+ chronic myeloid leukemia progenitors, preserving normal stem cells. Blood. 2012; 119:2335–2345.
Article52. Niu CC, Zhao C, Yang ZD, Zhang XL, Wu WR, Pan J, Zhao C, Li ZQ, Ding W, Yang Z, Si WK. Downregulation of γ-catenin inhibits CML cell growth and potentiates the response of CML cells to imatinib through β-catenin inhibition. Int J Mol Med. 2013; 31:453–458.
Article53. Jay J, Hammer A, Nestor-Kalinoski A, Diakonova M. JAK2 tyrosine kinase phosphorylates and is negatively regulated by centrosomal protein Ninein. Mol Cell Biol. 2015; 35:111–131.
Article54. Howng SL, Hsu HC, Cheng TS, Lee YL, Chang LK, Lu PJ, Hong YR. A novel ninein-interaction protein, CGI-99, blocks ninein phosphorylation by GSK3beta and is highly expressed in brain tumors. FEBS Lett. 2004; 566:162–168.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Janus activated kinase-3 protects against myocardial ischemia and reperfusion injury in mice
- Effect of Neurosteroid Modulation on Global Ischaemia-Reperfusion-Induced Cerebral Injury in Mice
- Effect of Mild Hypothermia on the Mitogen Activated Protein Kinases in Experimental Stroke
- Intracerebral Hematoma after Reperfusion Procedures in Cerebral Ischemia
- The Neuroprotective Effects of Woohwangcheongsim-won on Cerebral Ischemia following the Middle Cerebral Artery Occlusion in Rats