J Korean Neurosurg Soc.
2019 Nov;62(6):626-634. 10.3340/jkns.2019.0081.
Mouse Nerve Growth Factor Facilitates the Growth of Interspinal Schwannoma Cells by Activating NGF Receptors
- Affiliations
-
- 1School of Clinical Medicine, Xi’an Medical University, Xi’an, China. doclsy@163.com
- 2Department of Neurosurgery, Fuzhou Second Affiliated Hospital of Xiamen University, Fuzhou, China.
- 3Department of Otolaryngology, Second Affiliated Hospital of Xi’an Medical University, Xi’an, China.
- KMID: 2463654
- DOI: http://doi.org/10.3340/jkns.2019.0081
Abstract
OBJECTIVE
Nerve growth factor (NGF) is a member of the neurotrophic factor family and plays a vital role in the physiological processes of organisms, especially in the nervous system. Many recent studies have reported that NGF is also involved in the regulation of tumourigenesis by either promoting or suppressing tumor growth, which depends on the location and type of tumor. However, little is known regarding the effect of NGF on interspinal schwannoma (IS). In the present study, we aimed to explored whether mouse nerve growth factor (mNGF), which is widely used in the clinic, can influence the growth of interspinal schwannoma cells (ISCs) isolated from IS in vitro.
METHODS
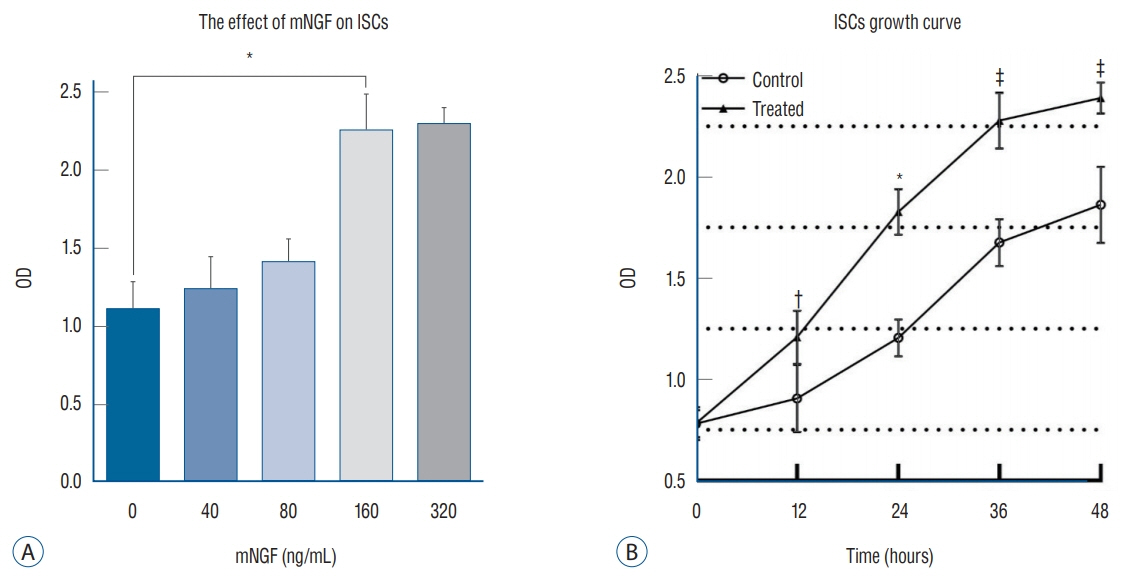
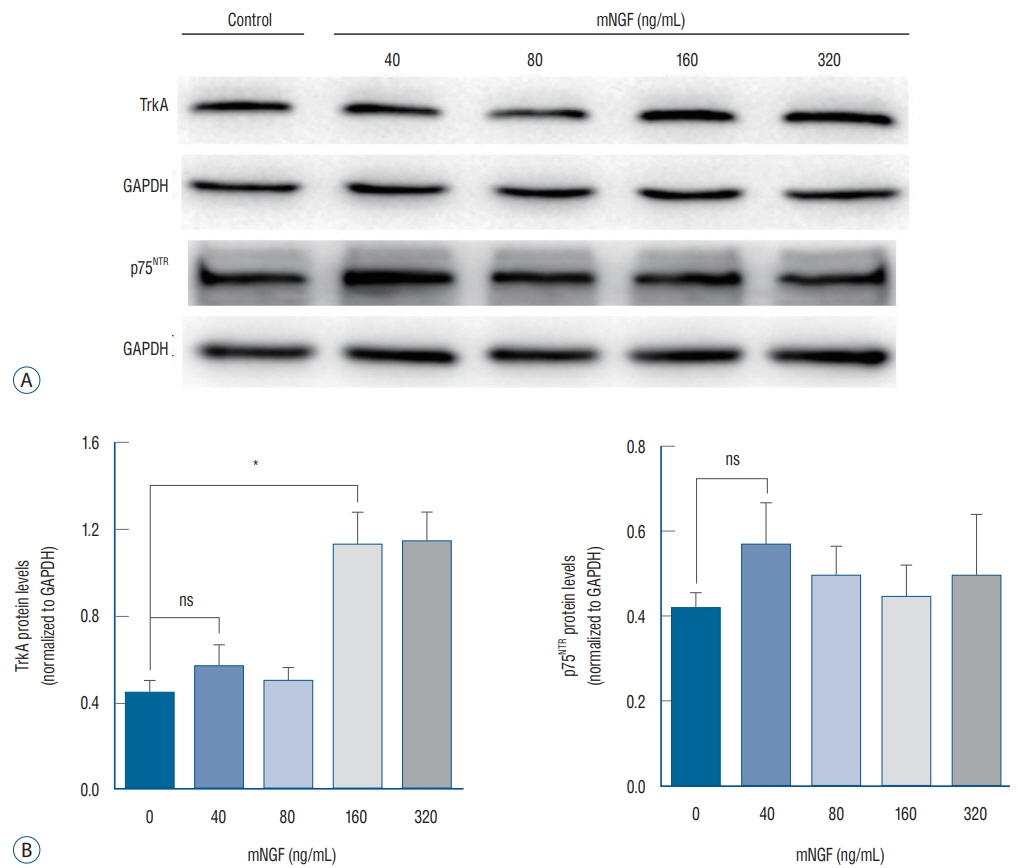
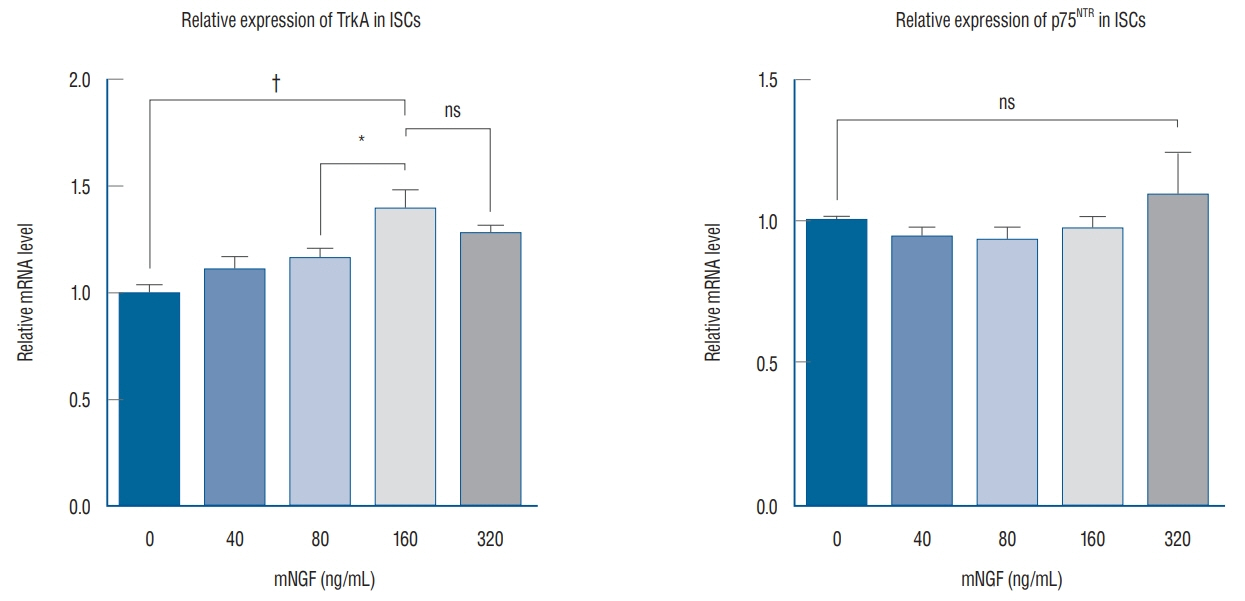
ISCs were isolated, cultured and identified by S-100 with immunofluorescence analysis. S-100-positive cells were divided into five groups, and separately cultured with various concentrations of mNGF (0 [phosphate buffered saline, PBS], 40, 80, 160, and 320 ng/mL) for 24 hours. Western blot and quantantive real time polymerase chain reaction (PCR) were applied to detect tyrosine kinase A (TrkA) receptor and p75 neurotrophin receptor (p75(NTR)) in each group. Crystal violet staining was selected to assess the effect of mNGF (160 ng/mL) on ISCs growth.
RESULTS
ISCs growth was enhanced by mNGF in a dose-dependent manner. The result of crystal violet staining revealed that it was significantly strengthened the cells growth kinetics when cultured with 160 ng/mL mNGF compared to PBS group. Western blot and quantantive real time PCR discovered that TrkA receptor and mRNA expression were both up-regualated under the condition of mNGF, expecially in 160 ng/mL, while the exoression of p75(NTR) demonstrated no difference among groups.
CONCLUSION
From these data, we conclude that exogenous mNGF can facilitate ISC growth by activating both TrkA receptor and p75(NTR). In addition, patients who are suffering from IS should not be administered mNGF in the clinic.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Fluorescent Antibody Technique
Gentian Violet
Humans
In Vitro Techniques
Kinetics
Mice*
Nerve Growth Factor*
Nervous System
Neurilemmoma*
Physiological Processes
Protein-Tyrosine Kinases
Real-Time Polymerase Chain Reaction
Receptor, Nerve Growth Factor*
Receptor, trkA
Receptors, Nerve Growth Factor*
RNA, Messenger
Gentian Violet
Nerve Growth Factor
Protein-Tyrosine Kinases
RNA, Messenger
Receptor, Nerve Growth Factor
Receptor, trkA
Receptors, Nerve Growth Factor
Figure
Reference
-
References
1. Ahmad I, Yue WY, Fernando A, Clark JJ, Woodson EA, Hansen MR. p75NTR is highly expressed in vestibular schwannomas and promotes cell survival by activating nuclear transcription factor kappa B. Glia. 62:1699–1712. 2014.
Article2. Chattopadhyay S, Shubayev VI. MMP-9 controls Schwann cell proliferation and phenotypic remodeling via IGF-1 and ErbB receptor-mediated activation of MEK/ERK pathway. Glia. 57:1316–1325. 2009.
Article3. Chou TT, Trojanowski JQ, Lee VM. A novel apoptotic pathway induced by nerve growth factor-mediated TrkA activation in medulloblastoma. J Biol Chem. 275:565–570. 2000.
Article4. Davidson B, Reich R, Lazarovici P, Nesland JM, Skrede M, Risberg B, et al. Expression and activation of the nerve growth factor receptor TrkA in serous ovarian carcinoma. Clin Cancer Res. 9:2248–2259. 2003.5. Friedman WJ. Proneurotrophins, seizures, and neuronal apoptosis. Neuroscientist. 16:244–252. 2010.
Article6. George DJ, Suzuki H, Bova GS, Isaacs JT. Mutational analysis of the TrkA gene in prostate cancer. Prostate. 36:172–180. 1998.
Article7. Gu R, Liu JB, Zhang Q, Liu GY, Zhu QS. MRI diagnosis of intradural extramedullary tumors. J Cancer Res Ther. 10:927–931. 2014.
Article8. Haase G, Pettmann B, Raoul C, Henderson CE. Signaling by death receptors in the nervous system. Curr Opin Neurobiol. 18:284–291. 2008.
Article9. Hellebrand EE, Varbiro G. Development of mitochondrial permeability transition inhibitory agents: a novel drug target. Drug Discov Ther. 4:54–61. 2010.10. Hondermarck H. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev. 23:357–365. 2012.
Article11. Jeon JH, Hwang HS, Jeong JH, Park SH, Moon JG, Kim CH. Spinal schwannoma; analysis of 40 cases. J Korean Neurosurg Soc. 43:135–138. 2008.
Article12. Johnson TV, Bull ND, Martin KR. Neurotrophic factor delivery as a protective treatment for glaucoma. Exp Eye Res. 93:196–203. 2011.
Article13. Khwaja F, Tabassum A, Allen J, Djakiew D. The p75(NTR) tumor suppressor induces cell cycle arrest facilitating caspase mediated apoptosis in prostate tumor cells. Biochem Biophys Res Commun. 341:1184–1192. 2006.
Article14. Krygier S, Djakiew D. The neurotrophin receptor p75NTR is a tumor suppressor in human prostate cancer. Anticancer Res. 21:3749–3755. 2001.15. Lagadec C, Meignan S, Adriaenssens E, Foveau B, Vanhecke E, Romon R, et al. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene. 28:1960–1970. 2009.
Article16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. 2001.
Article17. Makkerh JP, Ceni C, Auld DS, Vaillancourt F, Dorval G, Barker PA. p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 6:936–941. 2005.
Article18. Martinez-Glez V, Franco-Hernandez C, Alvarez L, de Campos JM., Isla A, Vaquero J, et al. Meningiomas and schwannomas: molecular subgroup classification found by expression arrays. Int J Oncol. 34:493–504. 2009.
Article19. McGregor LM, McCune BK, Graff JR, McDowell PR, Romans KE, Yancopoulos GD, et al. Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci U S A. 96:4540–4545. 1999.
Article20. Miknyoczki SJ, Dionne CA, Klein-Szanto AJ, Ruggeri BA. The novel Trk receptor tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits antitumor efficacy against human pancreatic carcinoma (Panc1) xenograft growth and in vivo invasiveness. Ann N Y Acad Sci. 880:252–262. 1999.
Article21. Mitsiadis TA, Pagella P. Expression of nerve growth factor (NGF), TrkA, and p75(NTR) in developing human fetal teeth. Front Physiol. 7:338. 2016.
Article22. Mizumura K, Murase S. Role of nerve growth factor in pain. Handb Exp Pharmacol. 227:57–77. 2015.
Article23. Murray SS, Perez P, Lee R, Hempstead BL, Chao MV. A novel p75 neurotrophin receptor-related protein, NRH2, regulates nerve growth factor binding to the TrkA receptor. J Neurosci. 24:2742–2749. 2004.
Article24. Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 328:847–854. 1993.
Article25. Ricci A, Greco S, Mariotta S, Felici L, Bronzetti E, Cavazzana A, et al. Neurotrophins and neurotrophin receptors in human lung cancer. Am J Respir Cell Mol Biol. 25:439–446. 2001.
Article26. Salehi AH, Xanthoudakis S, Barker PA. NRAGE, a p75 neurotrophin receptor-interacting protein, induces caspase activation and cell death through a JNK-dependent mitochondrial pathway. J Biol Chem. 277:48043–48050. 2002.
Article27. Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 846:1–12. 2012.
Article28. Thoenen H, Barde YA. Physiology of nerve growth factor. Physiol Rev. 60:1284–1335. 1980.
Article29. Tsunoda S, Okumura T, Ito T, Mori Y, Soma T, Watanabe G, et al. Significance of nerve growth factor overexpression and its autocrine loop in oesophagealsquamous cell carcinoma. Br J Cancer. 95:322–330. 2006.
Article30. Ullrich A, Gray A, Berman C, Dull TJ. Human β-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 303:821–825. 1983.
Article31. Varon S, Nomura J, Shooter EM. The isolation of the mouse nerve growth factor protein in a high molecular weight form. Biochemistry. 6:2202–2209. 1967.
Article32. Wadhwa S, Nag TC, Jindal A, Kushwaha R, Mahapatra AK, Sarkar C. Expression of the neurotrophin receptors Trk A and Trk B in adult human astrocytoma and glioblastoma. J Biosci. 28:181–188. 2003.
Article33. Wang X, Ying H, Zhou Z, Hu C, Eisbruch A. Successful treatment of radiation-induced temporal lobe necrosis with mouse nerve growth factor. J Clin Oncol. 29:e166–e168. 2011.
Article34. Yang T, Wu L, Deng X, Yang C, Xu Y. Clinical features and surgical outcomes of intramedullary schwannomas. Acta Neurochir (Wien). 156:1789–1797. 2014.
Article35. Zhang K, Chen S, Lin J, Zhang Y, Yu W, Liu S, et al. Primary culture of human interspinal schwannoma. J Biomater Tissue Eng. 6:263–269. 2016.
Article36. Zhu ZW, Friess H, Wang L, Bogardus T, Korc M, Kleeff J, et al. Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clin Cancer Res. 7:105–112. 2001.37. Zong S, Zeng G, Xiong C, Wei B. Treatment results in the differential surgery of intradural extramedullary schwannoma of 110 cases. PLoS One. 8:e63867. 2013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeting nerve growth factor for pain relief: pros and cons
- Effect of Nerve Growth Factor on Cultured Rat Schwann Cells in Hyperglycemic Condition
- A Correlation between Peripheral and Penile Serum Levels of Nerve Growth Factor in Healthy potent Controls
- Effect of TrkA and p75NTR on the Survival of Neurons in the Cultured Organotypic Hippocampal Slice
- Role of Nerve Growth Factor in Overactive Bladder