Korean J Pain.
2024 Oct;37(4):288-298. 10.3344/kjp.24235.
Targeting nerve growth factor for pain relief: pros and cons
- Affiliations
-
- 1Department of Biotechnology and Genetic Engineering, Faculty of Science, Philadelphia University, Amman, Jordan
- KMID: 2559691
- DOI: http://doi.org/10.3344/kjp.24235
Abstract
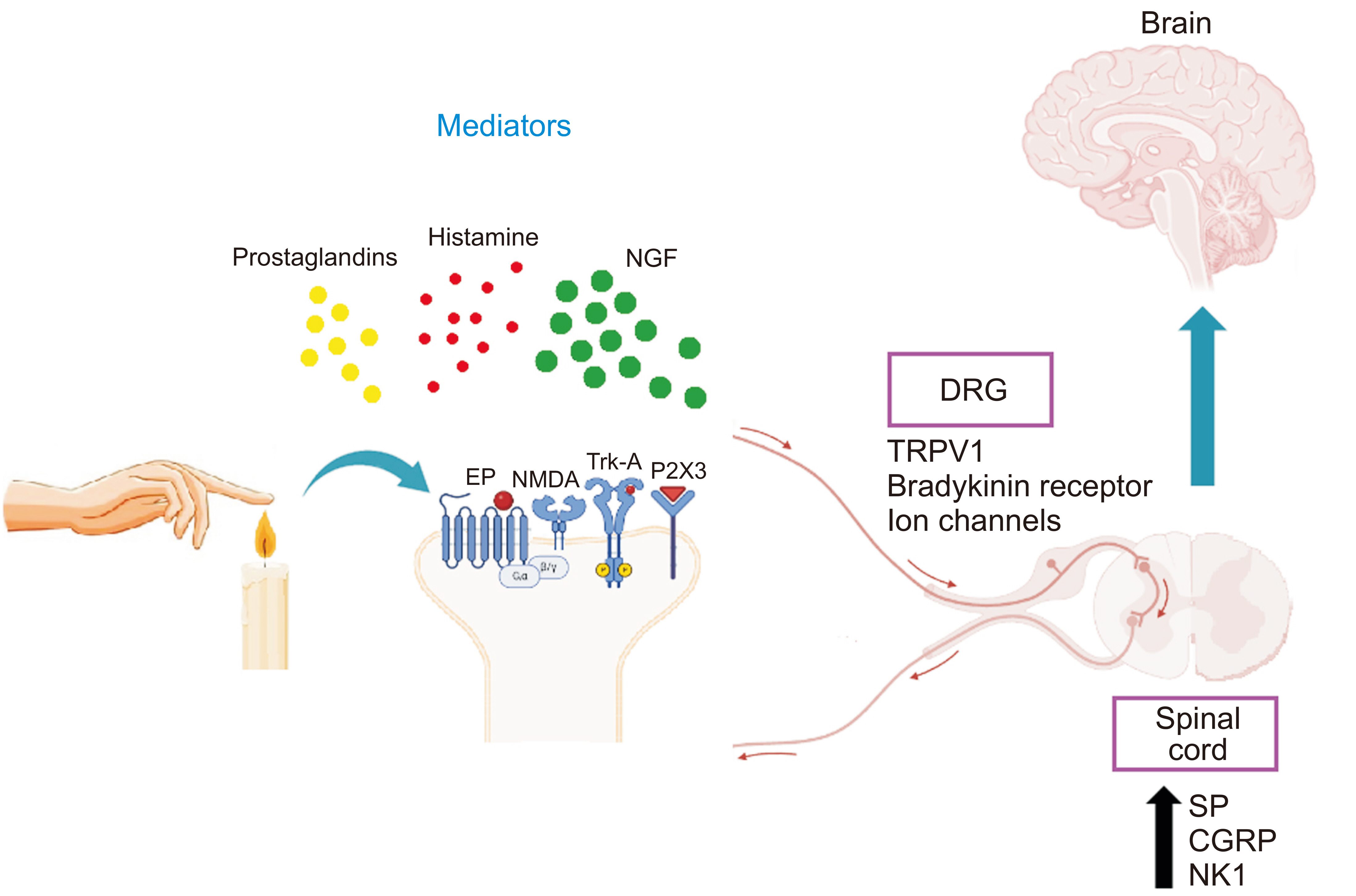
- Nerve growth factor (NGF) is a neurotrophic protein that has crucial roles in survival, growth and differentiation. It is expressed in neuronal and non-neuronal tissues. NGF exerts its effects via two types of receptors including the high affinity receptor, tropomyosin receptor kinase A and the low affinity receptor p75 neurotrophin receptor highlighting the complex signaling pathways that underlie the roles of NGF. In pain perception and transmission, multiple studies shed light on the effects of NGF on different types of pain including inflammatory, neuropathic, cancer and visceral pain. Also, the binding of NGF to its receptors increases the availability of many nociceptive receptors such as transient receptor potential vanilloid 1, transient receptor potential ankyrin 1, N-methyl-D-aspartic acid, and P2X purinoceptor 3 as well as nociceptive transmitters such as substance P and calcitonin gene-related peptide. The role of NGF in pain has been documented in pre-clinical and clinical studies. This review aims to shed light on the role of NGF and its signaling in different types of pain.
Keyword
Figure
Reference
-
1. Levi-Montalcini R, Angeletti PU. 1963; Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol. 6:653–9. DOI: 10.1016/0012-1606(63)90149-0. PMID: 13930092.2. Chang DS, Hsu E, Hottinger DG, Cohen SP. 2016; Anti-nerve growth factor in pain management: current evidence. J Pain Res. 9:373–83. DOI: 10.2147/JPR.S89061. PMID: 27354823. PMCID: PMC4908933.3. Yuan H, Du S, Chen L, Xu X, Wang Y, Ji F. 2020; Hypomethylation of nerve growth factor (NGF) promotes binding of C/EBPα and contributes to inflammatory hyperalgesia in rats. J Neuroinflammation. 17:34. DOI: 10.1186/s12974-020-1711-1. PMID: 31980031. PMCID: PMC6982391.4. Bennett DL. 2001; Neurotrophic factors: important regulators of nociceptive function. Neuroscientist. 7:13–7. DOI: 10.1177/107385840100700105. PMID: 11486340.5. Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, et al. 2006; Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 27:85–91. DOI: 10.1016/j.tips.2005.12.001. PMID: 16376998.6. Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, et al. 1994; Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 76:1001–11. DOI: 10.1016/0092-8674(94)90378-6. PMID: 8137419.7. Schmelz M, Mantyh P, Malfait AM, Farrar J, Yaksh T, Tive L, et al. 2019; Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain. 160:2210–20. DOI: 10.1097/j.pain.0000000000001625. PMID: 31145219. PMCID: PMC6756297.8. Osikowicz M, Longo G, Allard S, Cuello AC, Ribeiro-da-Silva A. 2013; Inhibition of endogenous NGF degradation induces mechanical allodynia and thermal hyperalgesia in rats. Mol Pain. 9:37. Erratum in: Mol Pain 2013; 9: 55. DOI: 10.1186/1744-8069-9-37. PMID: 23889761. PMCID: PMC3737061.9. Price TJ, Flores CM. 2007; Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 8:263–72. DOI: 10.1016/j.jpain.2006.09.005. PMID: 17113352. PMCID: PMC1899162.10. Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, et al. 2008; Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol. 128:2031–40. DOI: 10.1038/jid.2008.21. PMID: 18305571.11. Ricci A, Salvucci C, Castelli S, Carraturo A, de Vitis C, D'Ascanio M. 2022; Adenocarcinomas of the lung and neurotrophin system: a review. Biomedicines. 10:2531. DOI: 10.3390/biomedicines10102531. PMID: 36289793. PMCID: PMC9598928.12. Hirose M, Kuroda Y, Murata E. 2016; NGF/TrkA signaling as a therapeutic target for pain. Pain Pract. 16:175–82. DOI: 10.1111/papr.12342. PMID: 26452158.13. Wise BL, Seidel MF, Lane NE. 2021; The evolution of nerve growth factor inhibition in clinical medicine. Nat Rev Rheumatol. 17:34–46. DOI: 10.1038/s41584-020-00528-4. PMID: 33219344.14. Denk F, Bennett DL, McMahon SB. 2017; Nerve growth factor and pain mechanisms. Annu Rev Neurosci. 40:307–25. DOI: 10.1146/annurev-neuro-072116-031121. PMID: 28441116.15. Zhang X, Huang J, McNaughton PA. 2005; NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 24:4211–23. DOI: 10.1038/sj.emboj.7600893. PMID: 16319926. PMCID: PMC1356334.16. Oo WM, Hunter DJ. 2021; Nerve growth factor (NGF) inhibitors and related agents for chronic musculoskeletal pain: a comprehensive review. BioDrugs. 35:611–41. DOI: 10.1007/s40259-021-00504-8. PMID: 34807432.17. Barker PA, Mantyh P, Arendt-Nielsen L, Viktrup L, Tive L. 2020; Nerve growth factor signaling and its contribution to pain. J Pain Res. 13:1223–41. DOI: 10.2147/JPR.S247472. PMID: 32547184. PMCID: PMC7266393.18. Vera DB, Fredes AN, Garrido MP, Romero C. 2021; Role of mitochondria in interplay between NGF/TRKA, miR-145 and possible therapeutic strategies for epithelial ovarian cancer. Life (Basel). 12:8. DOI: 10.3390/life12010008. PMID: 35054401. PMCID: PMC8779980.19. Iftinca M, Defaye M, Altier C. 2021; TRPV1-targeted drugs in development for human pain conditions. Drugs. 81:7–27. DOI: 10.1007/s40265-020-01429-2. PMID: 33165872.20. Shane Anderson A, Loeser RF. 2010; Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 24:15–26. DOI: 10.1016/j.berh.2009.08.006. PMID: 20129196. PMCID: PMC2818253.21. Molloy NH, Read DE, Gorman AM. 2011; Nerve growth factor in cancer cell death and survival. Cancers (Basel). 3:510–30. DOI: 10.3390/cancers3010510. PMID: 24212627. PMCID: PMC3756375.22. Seidel MF, Herguijuela M, Forkert R, Otten U. 2010; Nerve growth factor in rheumatic diseases. Semin Arthritis Rheum. 40:109–26. DOI: 10.1016/j.semarthrit.2009.03.002. PMID: 19481238.23. Banks BE, Vernon CA, Warner JA. 1984; Nerve growth factor has anti-inflammatory activity in the rat hindpaw oedema test. Neurosci Lett. 47:41–5. DOI: 10.1016/0304-3940(84)90383-5. PMID: 6462528.24. Gigante A, Bevilacqua C, Pagnotta A, Manzotti S, Toesca A, Greco F. 2003; Expression of NGF, Trka and p75 in human cartilage. Eur J Histochem. 47:339–44. DOI: 10.4081/844. PMID: 14706929.25. Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, et al. 1994; Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 368:246–9. DOI: 10.1038/368246a0. PMID: 8145823.26. Schnitzer TJ, Marks JA. 2015; A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage. 23 Suppl 1:S8–17. DOI: 10.1016/j.joca.2014.10.003. PMID: 25527221.27. McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, et al. 2010; Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 149:386–92. DOI: 10.1016/j.pain.2010.03.002. PMID: 20350782.28. Voga M, Majdic G. 2022; Articular cartilage regeneration in veterinary medicine. Adv Exp Med Biol. 1401:23–55. DOI: 10.1007/5584_2022_717. PMID: 35733035.29. von Loga IS, El-Turabi A, Jostins L, Miotla-Zarebska J, Mackay-Alderson J, Zeltins A, et al. 2019; Active immunisation targeting nerve growth factor attenuates chronic pain behaviour in murine osteoarthritis. Ann Rheum Dis. 78:672–5. DOI: 10.1136/annrheumdis-2018-214489. PMID: 30862648. PMCID: PMC6517802.30. Aso K, Izumi M, Okanoue Y, Ikeuchi M. 2020; Effects of intraarticular injection of anti-nerve growth factor neutralizing antibody on pain in osteoarthritis rat. Int J Pain Relief. 4:1–5.31. Dakin P, DiMartino SJ, Gao H, Maloney J, Kivitz AJ, Schnitzer TJ, et al. 2019; The efficacy, tolerability, and joint safety of fasinumab in osteoarthritis pain: a phase IIb/III double-blind, placebo-controlled, randomized clinical trial. Arthritis Rheumatol. 71:1824–34. DOI: 10.1002/art.41012. PMID: 31207169. PMCID: PMC6900077.32. Miyagi M, Ishikawa T, Kamoda H, Suzuki M, Inoue G, Sakuma Y, et al. 2017; Efficacy of nerve growth factor antibody in a knee osteoarthritis pain model in mice. BMC Musculoskelet Disord. 18:428. DOI: 10.1186/s12891-017-1792-x. PMID: 29100502. PMCID: PMC5670727.33. Xu L, Nwosu LN, Burston JJ, Millns PJ, Sagar DR, Mapp PI, et al. 2016; The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthritis Cartilage. 24:1587–95. DOI: 10.1016/j.joca.2016.05.015. PMID: 27208420. PMCID: PMC5009895.34. Majuta LA, Guedon JG, Mitchell SAT, Ossipov MH, Mantyh PW. 2017; Anti-nerve growth factor therapy increases spontaneous day/night activity in mice with orthopedic surgery-induced pain. Pain. 158:605–17. DOI: 10.1097/j.pain.0000000000000799. PMID: 28301858. PMCID: PMC5370196.35. LaBranche TP, Bendele AM, Omura BC, Gropp KE, Hurst SI, Bagi CM, et al. 2017; Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann Rheum Dis. 76:295–302. DOI: 10.1136/annrheumdis-2015-208913. PMID: 27381034. PMCID: PMC5264211.36. Huang H, Shank G, Ma L, Tallents RH, Kyrkanides S. 2013; Nerve growth factor induced after temporomandibular joint inflammation decelerates chondrocyte differentiation. Oral Dis. 19:604–10. DOI: 10.1111/odi.12045. PMID: 23231346.37. Mammoto T, Seerattan RA, Paulson KD, Leonard CA, Bray RC, Salo PT. 2008; Nerve growth factor improves ligament healing. J Orthop Res. 26:957–64. DOI: 10.1002/jor.20615. PMID: 18302239.38. Pecchi E, Priam S, Gosset M, Pigenet A, Sudre L, Laiguillon MC, et al. 2014; Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in oa pain. Osteoarthritis Cartilage. 22:S23. DOI: 10.1016/j.joca.2014.02.065.39. Johnson AC, Farmer AD, Ness TJ, Greenwood-Van Meerveld B. 2020; Critical evaluation of animal models of visceral pain for therapeutics development: a focus on irritable bowel syndrome. Neurogastroenterol Motil. 32:e13776. DOI: 10.1111/nmo.13776. PMID: 31833625. PMCID: PMC7890461.40. Lane NE, Corr M. 2017; Osteoarthritis in 2016: anti-NGF treatments for pain - two steps forward, one step back? Nat Rev Rheumatol. 13:76–8. DOI: 10.1038/nrrheum.2016.224. PMID: 28119540.41. Meng F, Li H, Feng H, Long H, Yang Z, Li J, et al. 2022; Efficacy and safety of biologic agents for the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials. Ther Adv Musculoskelet Dis. 14:1759720X221080377. DOI: 10.1177/1759720X221080377. PMID: 35282570. PMCID: PMC8908403.42. Pallav M, Zaripova L, Tazhibaeva D, Kabdualieva N. 2022; POS1126 clinical efficacy and safety of monoclonal antibody against nerve growth factor and fibroblast growth factor-18 therapy of osteoarthritis. Ann Rheum Dis. 81:892. DOI: 10.1136/annrheumdis-2022-eular.3584.43. Leite VF, Buehler AM, El Abd O, Benyamin RM, Pimentel DC, Chen J, et al. 2014; Anti-nerve growth factor in the treatment of low back pain and radiculopathy: a systematic review and a meta-analysis. Pain Physician. 17:E45–60. DOI: 10.36076/ppj.2014/17/E45. PMID: 24452657.44. Markman JD, Bolash RB, McAlindon TE, Kivitz AJ, Pombo-Suarez M, Ohtori S, et al. 2020; Tanezumab for chronic low back pain: a randomized, double-blind, placebo- and active-controlled, phase 3 study of efficacy and safety. Pain. 161:2068–78. DOI: 10.1097/j.pain.0000000000001928. PMID: 32453139. PMCID: PMC7431140.45. Yang S, Huang Y, Ye Z, Li L, Zhang Y. 2020; The efficacy of nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: a meta-analysis. Front Pharmacol. 11:817. DOI: 10.3389/fphar.2020.00817. PMID: 33981217. PMCID: PMC8108005.46. Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, et al. 2011; Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 152:2248–58. DOI: 10.1016/j.pain.2011.05.003. PMID: 21696889.47. Kivitz AJ, Gimbel JS, Bramson C, Nemeth MA, Keller DS, Brown MT, et al. 2013; Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 154:1009–21. DOI: 10.1016/j.pain.2013.03.006. PMID: 23628600.48. Reed NR, Reed WR, Syrett M, Richey ML, Frolov A, Little JW. 2022; Somatosensory behavioral alterations in a NGF-induced persistent low back pain model. Behav Brain Res. 418:113617. DOI: 10.1016/j.bbr.2021.113617. PMID: 34606776.49. Peach CJ, Tonello R, Gomez K, Calderon-Rivera A, Bruni R, Bansia H, et al. 2024. Neuropilin-1 is a co-receptor for NGF and TrkA-evoked pain. bioRxiv 570398 [Preprint]. Available at: https://doi.org/10.1101/2023.12.06.570398. cited 2024 July 3. DOI: 10.1101/2023.12.06.570398.50. Mai L, Huang F, Zhu X, He H, Fan W. 2020; Role of nerve growth factor in orofacial pain. J Pain Res. 13:1875–82. DOI: 10.2147/JPR.S250030. PMID: 32801845. PMCID: PMC7399448.51. Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, et al. 2001; Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J Biol Chem. 276:17864–70. DOI: 10.1074/jbc.M010499200. PMID: 11359788.52. Verbeke S, Meignan S, Lagadec C, Germain E, Hondermarck H, Adriaenssens E, et al. 2010; Overexpression of p75(NTR) increases survival of breast cancer cells through p21(waf1). Cell Signal. 22:1864–73. DOI: 10.1016/j.cellsig.2010.07.014. PMID: 20667470.53. Lin H, Huang H, Yu Y, Chen W, Zhang S, Zhang Y. 2021; Nerve growth factor regulates liver cancer cell polarity and motility. Mol Med Rep. 23:288. DOI: 10.3892/mmr.2021.11927. PMID: 33649819. PMCID: PMC7905331.54. Buehlmann D, Ielacqua GD, Xandry J, Rudin M. 2019; Prospective administration of anti-nerve growth factor treatment effectively suppresses functional connectivity alterations after cancer-induced bone pain in mice. Pain. 160:151–9. DOI: 10.1097/j.pain.0000000000001388. PMID: 30161041.55. Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, et al. 2005; Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 115:128–41. DOI: 10.1016/j.pain.2005.02.022. PMID: 15836976.56. Jimenez-Andrade JM, Ghilardi JR, Castañeda-Corral G, Kuskowski MA, Mantyh PW. 2011; Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 152:2564–74. DOI: 10.1016/j.pain.2011.07.020. PMID: 21907491. PMCID: PMC3199350.57. Guedon JG, Longo G, Majuta LA, Thomspon ML, Fealk MN, Mantyh PW. 2016; Dissociation between the relief of skeletal pain behaviors and skin hypersensitivity in a model of bone cancer pain. Pain. 157:1239–47. DOI: 10.1097/j.pain.0000000000000514. PMID: 27186713. PMCID: PMC5142607.58. Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice ASC, et al. 2011; A new definition of neuropathic pain. Pain. 152:2204–5. DOI: 10.1016/j.pain.2011.06.017. PMID: 21764514.59. Foley KM. 2003; Opioids and chronic neuropathic pain. N Engl J Med. 348:1279–81. DOI: 10.1056/NEJMe030014. PMID: 12660393.60. Cirillo G, Cavaliere C, Bianco MR, De Simone A, Colangelo AM, Sellitti S, et al. 2010; Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol. 30:51–62. DOI: 10.1007/s10571-009-9430-2. PMID: 19585233.61. McArthur JC, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, et al. 2000; A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. AIDS Clinical Trials Group Team 291. Neurology. 54:1080–8. Erratum in: Neurology 2000; 55: 162. DOI: 10.1212/WNL.54.5.1080. PMID: 10720278.62. Schnitzer TJ, Khan A, Bessette L, Davignon I, Brown MT, Pixton G, et al. 2020; Onset and maintenance of efficacy of subcutaneous tanezumab in patients with moderate to severe osteoarthritis of the knee or hip: a 16-week dose-titration study. Semin Arthritis Rheum. 50:387–93. DOI: 10.1016/j.semarthrit.2020.03.004. PMID: 32252976.63. Dai WL, Yan B, Bao YN, Fan JF, Liu JH. 2020; Suppression of peripheral NGF attenuates neuropathic pain induced by chronic constriction injury through the TAK1-MAPK/NF-κB signaling pathways. Cell Commun Signal. 18:66. DOI: 10.1186/s12964-020-00556-3. PMID: 32312253. PMCID: PMC7171864.64. Dos Reis RC, Kopruszinski CM, Nones CF, Chichorro JG. 2016; Nerve growth factor induces facial heat hyperalgesia and plays a role in trigeminal neuropathic pain in rats. Behav Pharmacol. 27:528–35. DOI: 10.1097/FBP.0000000000000246. PMID: 27392124.65. Sainoh T, Sakuma Y, Miyagi M, Orita S, Yamauchi K, Inoue G, et al. 2014; Efficacy of anti-nerve growth factor therapy for discogenic neck pain in rats. Spine (Phila Pa 1976). 39:E757–62. DOI: 10.1097/BRS.0000000000000340. PMID: 24732837.66. Jasim H, Ghafouri B, Gerdle B, Hedenberg-Magnusson B, Ernberg M. 2020; Altered levels of salivary and plasma pain related markers in temporomandibular disorders. J Headache Pain. 21:105. DOI: 10.1186/s10194-020-01160-z. PMID: 32842964. PMCID: PMC7449051.67. Alhilou AM, Shimada A, Svensson CI, Svensson P, Ernberg M, Cairns BE, et al. 2021; Sex-related differences in response to masseteric injections of glutamate and nerve growth factor in healthy human participants. Sci Rep. 11:13873. DOI: 10.1038/s41598-021-93171-2. PMID: 34230516. PMCID: PMC8260580.68. Gao Y, Hu Z, Huang Y, Liu W, Ren C. 2022; Efficacy and safety of anti-nerve growth factor antibody therapy for hip and knee osteoarthritis: a meta-analysis. Orthop J Sports Med. 10:23259671221088590. DOI: 10.1177/23259671221088590. PMID: 35494494. PMCID: PMC9047886.69. Radithia D, Soebadi B, Parmadiati AE, Winias S. Rahmi. 2022; Nerve growth factor and S100B: molecular marker of neuroregeneration after injection of freeze-dried platelet rich plasma. J Oral Biol Craniofac Res. 12:570–4. DOI: 10.1016/j.jobcr.2022.07.006. PMID: 35957941. PMCID: PMC9361313.70. Boscato N, Exposto FG, Costa YM, Svensson P. 2022; Effect of standardized training in combination with masseter sensitization on corticomotor excitability in bruxer and control individuals: a proof of concept study. Sci Rep. 12:17469. DOI: 10.1038/s41598-022-21504-w. PMID: 36261447. PMCID: PMC9581922.71. Ye Y, Dang D, Zhang J, Viet CT, Lam DK, Dolan JC, et al. 2011; Nerve growth factor links oral cancer progression, pain, and cachexia. Mol Cancer Ther. 10:1667–76. DOI: 10.1158/1535-7163.MCT-11-0123. PMID: 21750223. PMCID: PMC3375020.72. Xu XJ, Zhang YL, Liu L, Pan L, Yao SK. 2017; Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: a preliminary explorative study. Aliment Pharmacol Ther. 45:100–14. DOI: 10.1111/apt.13848. PMID: 27862119.73. Coelho A, Oliveira R, Antunes-Lopes T, Cruz CD. 2019; Partners in crime: NGF and BDNF in visceral dysfunction. Curr Neuropharmacol. 17:1021–38. DOI: 10.2174/1570159X17666190617095844. PMID: 31204623. PMCID: PMC7052822.74. Li Q, Winston JH, Sarna SK. 2016; Noninflammatory upregulation of nerve growth factor underlies gastric hypersensitivity induced by neonatal colon inflammation. Am J Physiol Regul Integr Comp Physiol. 310:R235–42. DOI: 10.1152/ajpregu.00342.2015. PMID: 26608656. PMCID: PMC4796752.75. Barker K. 2022. Targeting pro-inflammatory mediators to treat visceral pain. [Doctoral dissertation]. University of Cambridge;Cambridge:76. Chen Y, Cheng J, Zhang Y, Chen JDZ, Seralu FM. 2021; Electroacupuncture at ST36 relieves visceral hypersensitivity via the NGF/TrkA/TRPV1 peripheral afferent pathway in a rodent model of post-inflammation rectal hypersensitivity. J Inflamm Res. 14:325–39. Erratum in: J Inflamm Res 2021; 14: 393. DOI: 10.2147/JIR.S285146. PMID: 33584100. PMCID: PMC7875081.77. Regmi B, Shah MK. 2020; Possible implications of animal models for the assessment of visceral pain. Animal Model Exp Med. 3:215–28. DOI: 10.1002/ame2.12130. PMID: 33024943. PMCID: PMC7529330.78. Liang D, Ren Y, Huang L, Jin S. 2022; A study on the mechanism of electroacupuncture to alleviate visceral pain and NGF expression. Comput Intell Neurosci. 2022:3755439. DOI: 10.1155/2022/3755439. PMID: 36275969. PMCID: PMC9586762.79. Jiao Y, Lin Y, Zheng J, Shi L, Zheng Y, Zhang Y, et al. 2022; Propionibacterium acnes contributes to low back pain via upregulation of NGF in TLR2-NF-κB/JNK or ROS pathway. Microbes Infect. 24:104980. DOI: 10.1016/j.micinf.2022.104980. PMID: 35430372.80. Rizzo RRN, Ferraro MC, Wewege MA, Cashin AG, Leake HB, O'Hagan ET, et al. 2022; Targeting neurotrophic factors for low back pain and sciatica: a systematic review and meta-analysis. Rheumatology (Oxford). 61:2243–54. DOI: 10.1093/rheumatology/keab785. PMID: 34677587.81. Delivanoglou N, Boziki M, Theotokis P, Kesidou E, Touloumi O, Dafi N, et al. 2020; Spatio-temporal expression profile of NGF and the two-receptor system, TrkA and p75NTR, in experimental autoimmune encephalomyelitis. J Neuroinflammation. 17:41. DOI: 10.1186/s12974-020-1708-9. PMID: 31996225. PMCID: PMC6990493.82. Yeh JF, Akinci A, Al Shaker M, Chang MH, Danilov A, Guileen R, et al. 2017; Monoclonal antibodies for chronic pain: a practical review of mechanisms and clinical applications. Mol Pain. 13:1744806917740233. DOI: 10.1177/1744806917740233. PMID: 29056066. PMCID: PMC5680940.83. Gwak YS, Bae JY, Jang JH, Yoon DM. 2003; Attenuation of spinal cord injury-induced hyperalgesia by administration of antibody to nerve growth factor in the rat. Korean J Pain. 16:7–13.84. El-Hashim AZ, Jaffal SM, Al-Rashidi FT, Luqmani YA, Akhtar S. 2013; Nerve growth factor enhances cough via a central mechanism of action. Pharmacol Res. 74:68–77. DOI: 10.1016/j.phrs.2013.05.003. PMID: 23742790.85. Minnone G, De Benedetti F, Bracci-Laudiero L. 2017; NGF and its receptors in the regulation of inflammatory response. Int J Mol Sci. 18:1028. DOI: 10.3390/ijms18051028. PMID: 28492466. PMCID: PMC5454940.86. Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. 2012; Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 13:790–8. DOI: 10.1016/j.jpain.2012.05.006. PMID: 22784777.87. Bernard NJ. 2019; NGF vaccine reduces pain. Nat Rev Rheumatol. 15:251. DOI: 10.1038/s41584-019-0213-y. PMID: 30923347.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Decisional Balances and the Process of Change in Smoking Cessation in Patients with Coronary Artery Diseases
- A study of the Stage of Change and Decisional balance: Exercise Acquisition, Smoking Cessation, Mammography Screening and Kegel's Exercise Acquisition in Korea
- The pros and cons of ultrasound-guided procedures in pain medicine
- Pros & Cons
- Comparing neuromodulation modalities involving the suprascapular nerve in chronic refractory shoulder pain: retrospective case series and literature review