Endocrinol Metab.
2019 Sep;34(3):302-313. 10.3803/EnM.2019.34.3.302.
Effects of Oxytocin on Cell Proliferation in a Corticotroph Adenoma Cell Line
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Institute of Evidence-based Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Cell Therapy and Tissue Engineering Center, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 5Department of Obstetrics and Gynecology, Yonsei University College of Medicine, Seoul, Korea.
- 6Yonsei Institute of Pharmaceutical Sciences, Yonsei University College of Pharmacy, Incheon, Korea.
- 7Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. ejlee423@yuhs.ac
- KMID: 2458633
- DOI: http://doi.org/10.3803/EnM.2019.34.3.302
Abstract
- BACKGROUND
Oxytocin (OXT) has been reported to act as a growth regulator in various tumor cells. However, there is a paucity of data on the influence of OXT on cell proliferation of corticotroph adenomas. This study aimed to examine whether OXT affects cell growth in pituitary tumor cell lines (AtT20 and GH3 cells) with a focus on corticotroph adenoma cells.
METHODS
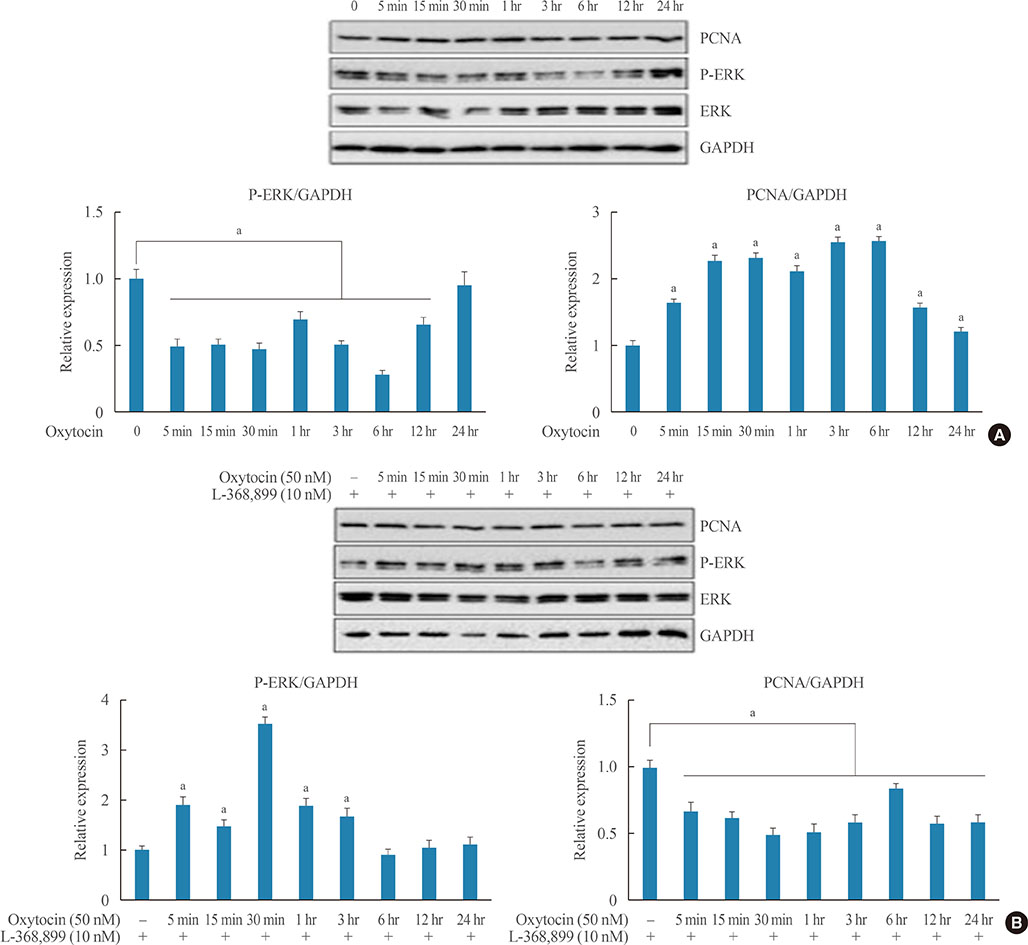
Reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay were conducted with AtT20 cells to confirm the effects of OXT on hormonal activity; flow cytometry was used to assess changes in the cell cycle after OXT treatment. Moreover, the impact of OXT on proliferating cell nuclear antigen (PCNA), nuclear factor κB, and mitogen-activated protein kinase signaling pathway was analyzed by Western blot.
RESULTS
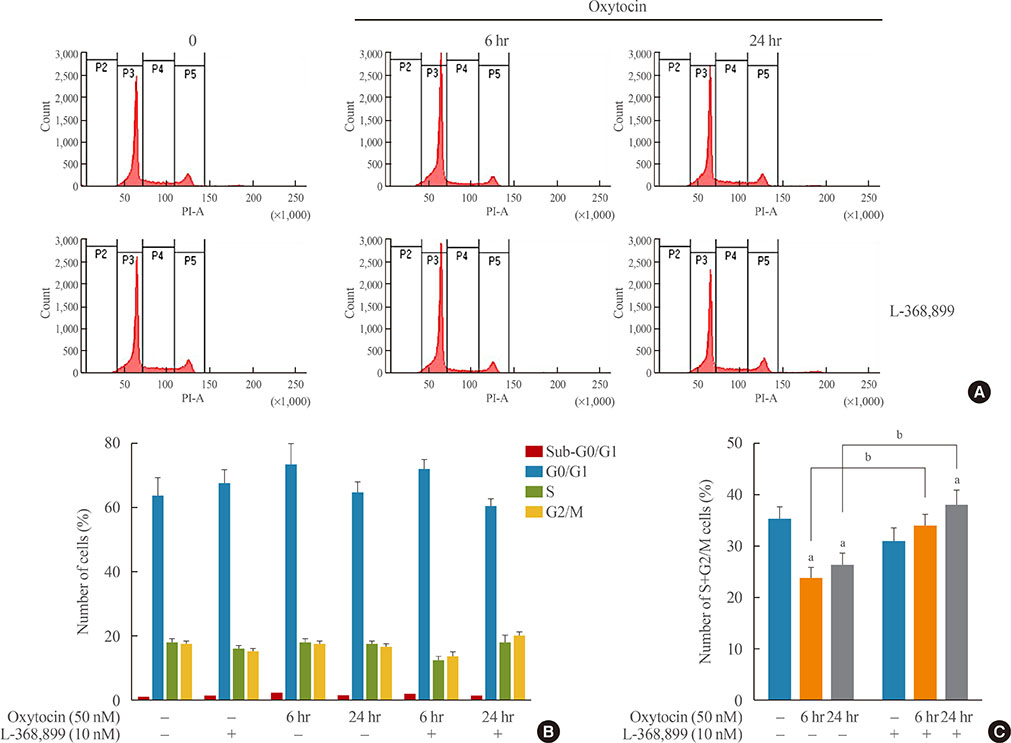
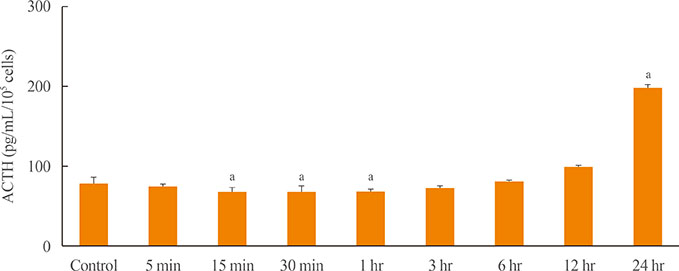
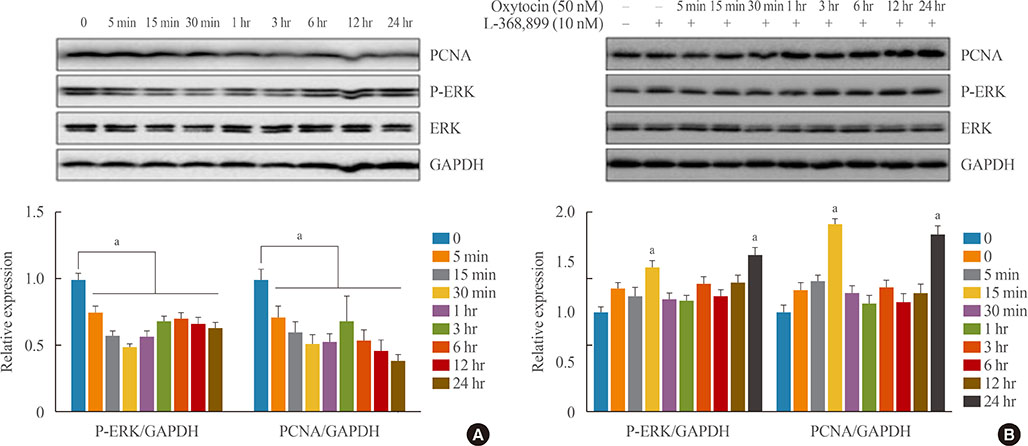
OXT treatment of 50 nM changed the gene expression of OXT receptor and pro-opiomelanocortin within a short time. In addition, OXT significantly reduced adrenocorticotropic hormone secretion within 1 hour. S and G2/M populations of AtT20 cells treated with OXT for 24 hours were significantly decreased compared to the control. Furthermore, OXT treatment decreased the protein levels of PCNA and phosphorylated extracellular-signal-regulated kinase (P-ERK) in AtT20 cells.
CONCLUSION
Although the cytotoxic effect of OXT in AtT20 cells was not definite, OXT may blunt cell proliferation of corticotroph adenomas by altering the cell cycle or reducing PCNA and P-ERK levels. Further research is required to investigate the role of OXT as a potential therapeutic target in corticotroph adenomas.
MeSH Terms
-
ACTH-Secreting Pituitary Adenoma*
Adrenocorticotropic Hormone
Blotting, Western
Cell Cycle
Cell Line*
Cell Proliferation*
Corticotrophs*
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
Gene Expression
Oxytocin*
Phosphotransferases
Pituitary Neoplasms
Polymerase Chain Reaction
Pro-Opiomelanocortin
Proliferating Cell Nuclear Antigen
Protein Kinases
Reverse Transcription
Adrenocorticotropic Hormone
Oxytocin
Phosphotransferases
Pro-Opiomelanocortin
Proliferating Cell Nuclear Antigen
Protein Kinases
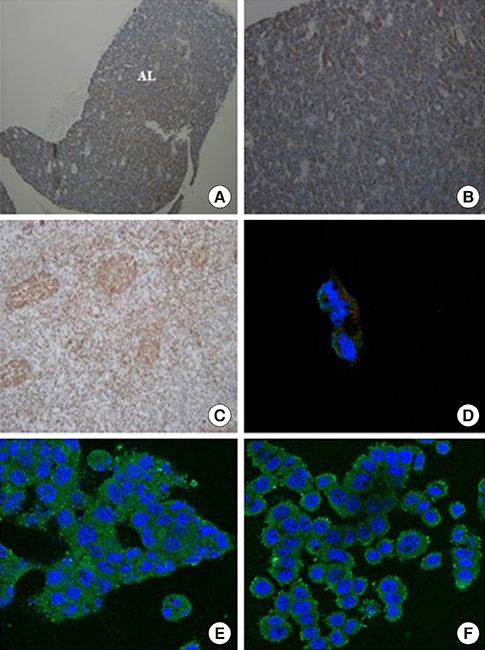
Figure
Reference
-
1. De Martin M, Pecori Giraldi F, Cavagnini F. Cushing's disease. Pituitary. 2006; 9:279–287.
Article2. Patil CG, Prevedello DM, Lad SP, Vance ML, Thorner MO, Katznelson L, et al. Late recurrences of Cushing's disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab. 2008; 93:358–362.
Article3. Sonino N, Zielezny M, Fava GA, Fallo F, Boscaro M. Risk factors and long-term outcome in pituitary-dependent Cushing's disease. J Clin Endocrinol Metab. 1996; 81:2647–2652.
Article4. Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, et al. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2008; 93:2454–2462.
Article5. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001; 81:629–683.6. Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007; 176:170–186.
Article7. Qian W, Zhu T, Tang B, Yu S, Hu H, Sun W, et al. Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. J Clin Endocrinol Metab. 2014; 99:4683–4689.
Article8. Breuil V, Panaia-Ferrari P, Fontas E, Roux C, Kolta S, Eastell R, et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: analysis of the OPUS cohort. J Clin Endocrinol Metab. 2014; 99:E634–E641.
Article9. Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biol Psychiatry. 2001; 50:609–613.
Article10. Kiss A, Mikkelsen JD. Oxytocin: anatomy and functional assignments. A minireview. Endocr Regul. 2005; 39:97–105.11. Yu Q, Ji R, Gao X, Fu J, Guo W, Song X, et al. Oxytocin is expressed by both intrinsic sensory and secretomotor neurons in the enteric nervous system of guinea pig. Cell Tissue Res. 2011; 344:227–237.
Article12. Amico JA, Tenicela R, Johnston J, Robinson AG. A time-dependent peak of oxytocin exists in cerebrospinal fluid but not in plasma of humans. J Clin Endocrinol Metab. 1983; 57:947–951.
Article13. Zingg HH, Laporte SA. The oxytocin receptor. Trends Endocrinol Metab. 2003; 14:222–227.
Article14. Cassoni P, Sapino A, Marrocco T, Chini B, Bussolati G. Oxytocin and oxytocin receptors in cancer cells and proliferation. J Neuroendocrinol. 2004; 16:362–364.
Article15. Imanieh MH, Bagheri F, Alizadeh AM, Ashkani-Esfahani S. Oxytocin has therapeutic effects on cancer, a hypothesis. Eur J Pharmacol. 2014; 741:112–123.
Article16. Cassoni P, Sapino A, Munaron L, Deaglio S, Chini B, Graziani A, et al. Activation of functional oxytocin receptors stimulates cell proliferation in human trophoblast and choriocarcinoma cell lines. Endocrinology. 2001; 142:1130–1136.17. Pequeux C, Breton C, Hendrick JC, Hagelstein MT, Martens H, Winkler R, et al. Oxytocin synthesis and oxytocin receptor expression by cell lines of human small cell carcinoma of the lung stimulate tumor growth through autocrine/paracrine signaling. Cancer Res. 2002; 62:4623–4629.18. Cassoni P, Fulcheri E, Carcangiu ML, Stella A, Deaglio S, Bussolati G. Oxytocin receptors in human adenocarcinomas of the endometrium: presence and biological significance. J Pathol. 2000; 190:470–477.
Article19. Morita T, Shibata K, Kikkawa F, Kajiyama H, Ino K, Mizutani S. Oxytocin inhibits the progression of human ovarian carcinoma cells in vitro and in vivo. Int J Cancer. 2004; 109:525–532.20. Petersson M. Opposite effects of oxytocin on proliferation of osteosarcoma cell lines. Regul Pept. 2008; 150:50–54.
Article21. Taylor AH, Ang VT, Jenkins JS, Silverlight JJ, Coombes RC, Luqmani YA. Interaction of vasopressin and oxytocin with human breast carcinoma cells. Cancer Res. 1990; 50:7882–7886.22. Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Oxytocin inhibits the proliferation of MDA-MB231 human breast-cancer cells via cyclic adenosine monophosphate and protein kinase A. Int J Cancer. 1997; 72:340–344.23. Cassoni P, Sapino A, Stella A, Fortunati N, Bussolati G. Presence and significance of oxytocin receptors in human neuroblastomas and glial tumors. Int J Cancer. 1998; 77:695–700.
Article24. Bakos J, Strbak V, Ratulovska N, Bacova Z. Effect of oxytocin on neuroblastoma cell viability and growth. Cell Mol Neurobiol. 2012; 32:891–896.
Article25. Whittington K, Connors B, King K, Assinder S, Hogarth K, Nicholson H. The effect of oxytocin on cell proliferation in the human prostate is modulated by gonadal steroids: implications for benign prostatic hyperplasia and carcinoma of the prostate. Prostate. 2007; 67:1132–1142.
Article26. Liu JW, Ben-Jonathan N. Prolactin-releasing activity of neurohypophysial hormones: structure-function relationship. Endocrinology. 1994; 134:114–118.
Article27. Gonzalez-Iglesias AE, Fletcher PA, Arias-Cristancho JA, Cristancho-Gordo R, Helena CV, Bertram R, et al. Direct stimulatory effects of oxytocin in female rat gonadotrophs and somatotrophs in vitro: comparison with lactotrophs. Endocrinology. 2015; 156:600–612.
Article28. Asa SL, Ezzat S. The pathogenesis of pituitary tumors. Annu Rev Pathol. 2009; 4:97–126.
Article29. Langlois F, McCartney S, Fleseriu M. Recent progress in the medical therapy of pituitary tumors. Endocrinol Metab (Seoul). 2017; 32:162–170.
Article30. Dworakowska D, Grossman AB. The molecular pathogenesis of corticotroph tumours. Eur J Clin Invest. 2012; 42:665–676.
Article31. Roussel-Gervais A, Bilodeau S, Vallette S, Berthelet F, Lacroix A, Figarella-Branger D, et al. Cooperation between cyclin E and p27(Kip1) in pituitary tumorigenesis. Mol Endocrinol. 2010; 24:1835–1845.
Article32. Jankowski M, Broderick TL, Gutkowska J. Oxytocin and cardioprotection in diabetes and obesity. BMC Endocr Disord. 2016; 16:34.
Article33. Breton C, Pechoux C, Morel G, Zingg HH. Oxytocin receptor messenger ribonucleic acid: characterization, regulation, and cellular localization in the rat pituitary gland. Endocrinology. 1995; 136:2928–2936.
Article34. Shibasaki T, Masui H. Effects of various neuropeptides on the secretion of proopiomelanocortin-derived peptides by a cultured pituitary adenoma causing Nelson's syndrome. J Clin Endocrinol Metab. 1982; 55:872–876.
Article35. Link H, Dayanithi G, Fohr KJ, Gratzl M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH-releasing factor or arginine vasopressin-induced ACTH secretion, respectively. Endocrinology. 1992; 130:2183–2191.
Article36. Gibbs DM. Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sci. 1984; 35:487–491.
Article37. Petersson M, Hulting AL, Uvnas-Moberg K. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neurosci Lett. 1999; 264:41–44.
Article38. Nakamura K, Fujiwara Y, Mizutani R, Sanbe A, Miyauchi N, Hiroyama M, et al. Effects of vasopressin V1b receptor deficiency on adrenocorticotropin release from anterior pituitary cells in response to oxytocin stimulation. Endocrinology. 2008; 149:4883–4891.
Article39. Page SR, Ang VT, Jackson R, White A, Nussey SS, Jenkins JS. The effect of oxytocin infusion on adenohypophyseal function in man. Clin Endocrinol (Oxf). 1990; 32:307–313.
Article40. Jafarzadeh N, Javeri A, Khaleghi M, Taha MF. Oxytocin improves proliferation and neural differentiation of adipose tissue-derived stem cells. Neurosci Lett. 2014; 564:105–110.
Article41. Dworakowska D, Wlodek E, Leontiou CA, Igreja S, Cakir M, Teng M, et al. Activation of RAF/MEK/ERK and PI3K/AKT/mTOR pathways in pituitary adenomas and their effects on downstream effectors. Endocr Relat Cancer. 2009; 16:1329–1338.
Article42. Guzzi F, Zanchetta D, Cassoni P, Guzzi V, Francolini M, Parenti M, et al. Localization of the human oxytocin receptor in caveolin-1 enriched domains turns the receptor-mediated inhibition of cell growth into a proliferative response. Oncogene. 2002; 21:1658–1667.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Problems Associated with I-125 Oxytocin Binding to Membrane Receptors
- Establishment and Characterization of a Murine Erythroleukemia Cell Line Stimulation B Cell Proliferation
- Effect of estrogen on growth hormone receptor expression of human periodontal ligament cell line
- A Case of Silent Corticotroph-cell Adenoma with Elevated Serum ACTH
- Roles of Dopamine in Proliferation of Gastric-Cancer Cells