Nutr Res Pract.
2019 Aug;13(4):279-285. 10.4162/nrp.2019.13.4.279.
Protective effect of Cordyceps militaris against hydrogen peroxide-induced oxidative stress in vitro
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, Busandaehak-ro 63 beon-gil 2, geumjeong-gu, Busan 46241, Korea. ejcho@pusan.ac.kr
- 2Department of Food Science, Gyeongnam National University of Science and Technology, Jinju 52725, Korea.
- 3Department of Medicinal Crop Research, National Institute of Horticultural and Herbal Science, Rural Development Administration, Eumseong 27709, Korea.
- KMID: 2453105
- DOI: http://doi.org/10.4162/nrp.2019.13.4.279
Abstract
- BACKGROUND/OBJECTIVES
Excessive production of reactive oxygen species (ROS) such as hydroxyl (·OH), nitric oxide (NO), and hydrogen peroxide (H2O2) is reported to induce oxidative stress. ROS generated by oxidative stress can potentially damage glial cells in the nervous system. Cordyceps militaris (CM), a kind of natural herb widely found in East Asia. In this study, we investigated the free radical scavenging activity of the CM extract and its neuroprotective effects in H2O2-induced C6 glial cells.
MATERIALS/METHODS
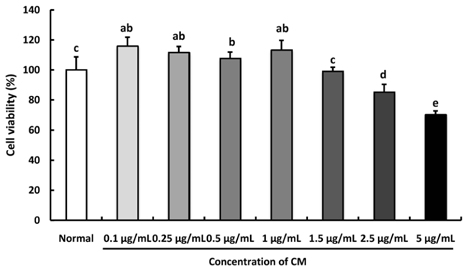
The ethanol extract of CM (100-1,000 µg/mL) was used to measure DPPH, ·OH, and NO radical scavenging activities. In addition, hydrogen peroxide (H2O2)-induced C6 glial cells were treated with CM at 0.5-2.5 µg/mL for measurement of cell viability, ROS production, and protein expression resulting from oxidative stress.
RESULTS
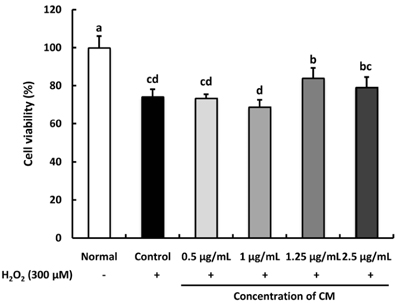
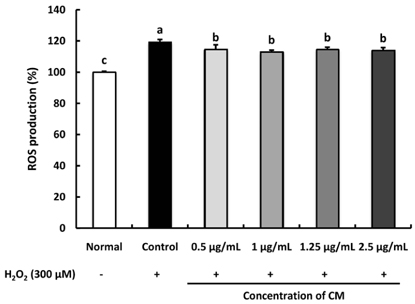
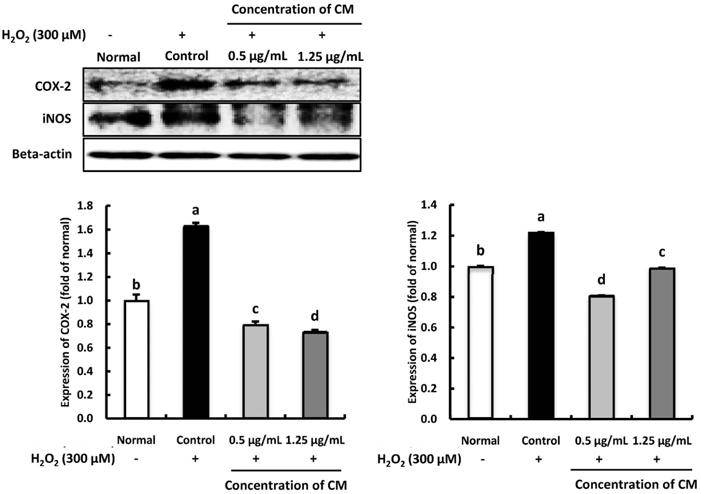
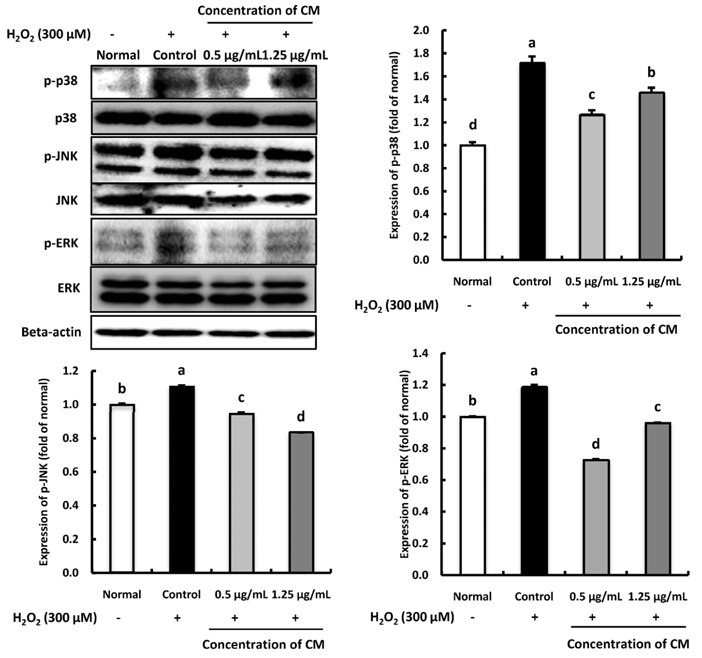
The CM extract showed high scavenging activities against DPPH, ·OH, and NO radicals at concentration of 1,000 µg/mL. Treatment of CM with H2O2-induced oxidative stress in C6 glial cells significantly increased cell viability, and decreased ROS production. Cyclooxygenase-2 and inducible nitric oxide synthase protein expression was down-regulated in CM-treated groups. In addition, the protein expression level of phospho-p38 mitogen-activated protein kinase (p-p38 MAPK), phospho-c-Jun N-terminal kinase (p-JNK), and phospho-extracellular regulated protein kinases (p-ERK) in H2O2-induced C6 glial cells was down-regulated upon CM administration.
CONCLUSION
CM exhibited radical scavenging activity and protective effect against H2O2 as indicated by the increased cell viability, decreased ROS production, down-regulation of inflammation-related proteins as well as p-p38, p-JNK, and p-ERK protein levels. Therefore, we suggest that CM could play the protective role from oxidative stress in glial cells.
MeSH Terms
-
Cell Survival
Cordyceps*
Cyclooxygenase 2
Down-Regulation
Ethanol
Far East
Free Radicals
Hydrogen Peroxide
Hydrogen*
In Vitro Techniques*
Nervous System
Neuroglia
Neuroprotective Agents
Nitric Oxide
Nitric Oxide Synthase Type II
Oxidative Stress*
Phosphotransferases
Protein Kinases
Reactive Oxygen Species
Cyclooxygenase 2
Ethanol
Free Radicals
Hydrogen
Hydrogen Peroxide
Neuroprotective Agents
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphotransferases
Protein Kinases
Reactive Oxygen Species
Figure
Reference
-
1. Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013; 60:1–4.
Article2. Juránek I, Bezek S. Controversy of free radical hypothesis: reactive oxygen species--cause or consequence of tissue injury? Gen Physiol Biophys. 2005; 24:263–278.3. Bayir H. Reactive oxygen species. Crit Care Med. 2005; 33:S498–S501.4. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012; 24:981–990.
Article5. Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007; 12:913–922.
Article6. Siddique YH, Beg T, Afzal M. Protective effect of ascorbic acid against oxidative damage induced by hydrogen peroxide in cultured human peripheral blood lymphocytes. Indian J Clin Biochem. 2009; 24:294–300.
Article7. Chandra K, Salman AS, Mohd A, Sweety R, Ali KN. Protection against FCA induced oxidative stress induced DNA damage as a model of arthritis and in vitro anti-arthritic potential of Costus speciosus rhizome extract. Inter J Pharm Phytochem Res. 2015; 7:383–389.8. Gille JJ, Joenje H. Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat Res. 1992; 275:405–414.
Article9. Spitz DR, Li GC, McCormick ML, Sun Y, Oberley LW. Stable H2O2-resistant variants of Chinese hamster fibroblasts demonstrate increases in catalase activity. Radiat Res. 1988; 114:114–124.
Article10. Rojkind M, Domínguez-Rosales JA, Nieto N, Greenwel P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol Life Sci. 2002; 59:1872–1891.
Article11. Minghetti L, Polazzi E, Nicolini A, Levi G. Opposite regulation of prostaglandin E2 synthesis by transforming growth factor-β1 and interleukin 10 in activated microglial cultures. J Neuroimmunol. 1998; 82:31–39.
Article12. Prajeeth CK, Huehn J, Stangel M. Regulation of neuroinflammatory properties of glial cells by T cell effector molecules. Neural Regen Res. 2018; 13:234–236.
Article13. Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004; 3:205–214.
Article14. Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005; 58:495–505.
Article15. Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. 2006; 3:327–337.
Article16. Li YZ, Ren S, Yan XT, Li HP, Li W, Zheng B, Wang Z, Liu YY. Improvement of Cisplatin-induced renal dysfunction by Schisandra chinensis stems via anti-inflammation and anti-apoptosis effects. J Ethnopharmacol. 2018; 217:228–237.
Article17. Choi YJ, Kim HS, Lee J, Chung J, Lee JS, Choi JS, Yoon TR, Kim HK, Chung HY. Down-regulation of oxidative stress and COX-2 and iNOS expressions by dimethyl lithospermate in aged rat kidney. Arch Pharm Res. 2014; 37:1032–1038.
Article18. Kumamoto H, Ooya K. Immunohistochemical detection of phosphorylated JNK, p38 MAPK, and ERK5 in ameloblastic tumors. J Oral Pathol Med. 2007; 36:543–549.
Article19. Wu XF, Zhang M, Bhandari B, Li Z. Effects of microwave-assisted pulse-spouted bed freeze-drying (MPSFD) on volatile compounds and structural aspects of Cordyceps militaris. J Sci Food Agric. 2018; 98:4634–4643.
Article20. Huang SJ, Huang FK, Li YS, Tsai SY. The quality improvement of solid-state fermentation with Cordyceps militaris by UVB irradiation. Food Technol Biotechnol. 2017; 55:445–453.
Article21. Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia. 2010; 81:961–968.
Article22. Chu HL, Chien JC, Duh PD. Protective effect of Cordyceps militaris against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Food Chem. 2011; 129:871–876.
Article23. Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 2005; 57:1509–1519.
Article24. Ramesh T, Yoo SK, Kim SW, Hwang SY, Sohn SH, Kim IW, Kim SK. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp Gerontol. 2012; 47:979–987.
Article25. Bawadekji A, Al Ali K, Al Ali M. A review of the bioactive compound and medicinal value of Cordyceps militaris. J North Basic Appl Sci. 2016; 347:1–3.
Article26. Li XT, Li HC, Li CB, Dou DQ, Gao MB. Protective effects on mitochondria and anti-aging activity of polysaccharides from cultivated fruiting bodies of Cordyceps militaris. Am J Chin Med. 2010; 38:1093–1106.
Article27. Lin R, Liu H, Wu S, Pang L, Jia M, Fan K, Jia S, Jia L. Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int J Biol Macromol. 2012; 51:153–157.
Article28. Park JM, Lee JS, Lee KR, Ha SJ, Hong EK. Cordyceps militaris extract protects human dermal fibroblasts against oxidative stress-induced apoptosis and premature senescence. Nutrients. 2014; 6:3711–3726.
Article29. Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T. Effects of the interaction of tannins with co-existing substances. VI.: effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-Diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull (Tokyo). 1989; 37:2016–2021.
Article30. Chung SK, Osawa T, Kawakishi S. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra). Biosci Biotechnol Biochem. 1997; 61:118–123.
Article31. Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994; 201:748–755.
Article32. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article33. Ma WW, Hou CC, Zhou X, Yu HL, Xi YD, Ding J, Zhao X, Xiao R. Genistein alleviates the mitochondria-targeted DNA damage induced by β-amyloid peptides 25–35 in C6 glioma cells. Neurochem Res. 2013; 38:1315–1323.
Article34. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958; 181:1199–1200.
Article35. Hayyan M, Hashim MA, AlNashef IM. Superoxide ion: generation and chemical implications. Chem Rev. 2016; 116:3029–3085.
Article36. Patel Rajesh M, Patel Natvar J. In vitro antioxidant activity of coumarin compounds by DPPH, Superoxide and nitric oxide free radical scavenging methods. J Adv Pharm Technol Res. 2011; 1:52–68.37. Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998; 75:199–212.
Article38. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39:44–84.
Article39. Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004; 55:373–399.
Article40. Dong CH, Yang T, Lian T. A comparative study of the antimicrobial, antioxidant, and cytotoxic activities of methanol extracts from fruit bodies and fermented mycelia of caterpillar medicinal mushroom Cordyceps militaris (Ascomycetes). Int J Med Mushrooms. 2014; 16:485–495.
Article41. Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980; 286:736–737.
Article42. Sadigh-Eteghad S, Majdi A, Mahmoudi J, Golzari SE, Talebi M. Astrocytic and microglial nicotinic acetylcholine receptors: an overlooked issue in Alzheimer's disease. J Neural Transm (Vienna). 2016; 123:1359–1367.
Article43. Choi EO, Jeong JW, Park C, Hong SH, Kim GY, Hwang HJ, Cho EJ, Choi YH. Baicalein protects C6 glial cells against hydrogen peroxide-induced oxidative stress and apoptosis through regulation of the Nrf2 signaling pathway. Int J Mol Med. 2016; 37:798–806.
Article44. Valori CF, Brambilla L, Martorana F, Rossi D. The multifaceted role of glial cells in amyotrophic lateral sclerosis. Cell Mol Life Sci. 2014; 71:287–297.
Article45. Puves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia AS, White LE. Neuroscience. 5th ed. Sunderland (MA): Sinauer Associates, Inc.;2012. p. 560–580.46. McGeer PL, McGeer EG. Glial cell reactions in neurodegenerative diseases: pathophysiology and therapeutic interventions. Alzheimer Dis Assoc Disord. 1998; 12:Suppl 2. S1–S6.
Article47. Yu H, Liu Z, Zhou H, Dai W, Chen S, Shu Y, Feng J. JAK-STAT pathway modulates the roles of iNOS and COX-2 in the cytoprotection of early phase of hydrogen peroxide preconditioning against apoptosis induced by oxidative stress. Neurosci Lett. 2012; 529:166–171.
Article48. Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004; 63:901–910.
Article49. Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1996; 93:15069–15074.
Article50. Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004; 45:170–179.
Article51. Kwon SH, Kim JA, Hong SI, Jung YH, Kim HC, Lee SY, Jang CG. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 2011; 58:533–541.
Article52. Choi YH, Kim GY, Lee HH. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des Devel Ther. 2014; 8:1941–1953.53. Shin JW, Seol IC, Son CG. Interpretation of animal dose and human equivalent dose for drug development. J Korean Orient Med. 2010; 31:1–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- Effect of Ascorbic Acid Against the Oxidative Stress-Induced Cellular Senescence in Trabecular Meshwork Cells
- Role of Ascorbic Acid Against the Oxidative Stress in Trabecular Meshwork Cells
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell
- Overexpressed Mitochondrial Thioredoxin Protects PC12 Cells from Hydrogen Peroxide and Serum-deprivation