Acute Crit Care.
2019 May;34(2):133-140. 10.4266/acc.2019.00507.
The effects of BMS-470539 on lipopolysaccharide-induced acute lung injury
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University Medical School and Hospital, Gwangju, Korea. shkwak@jnu.ac.kr
- 2Brain Korea 21 Project, Center for Creative Biomedical Scientists at Chonnam National University, Gwangju, Korea.
- KMID: 2449367
- DOI: http://doi.org/10.4266/acc.2019.00507
Abstract
- BACKGROUND
Overactivation of inflammatory cells, including macrophages and neutrophils, is associated with acute lung injury. BMS-470539 is a selective agonist of melanocortin 1 receptor, which triggers the inhibition of proinflammatory responses, suppressing neutrophil infiltration and protecting tissue. This study evaluated the effects of BMS-470539 on lipopolysaccharide-induced acute lung injury in a mouse model.
METHODS
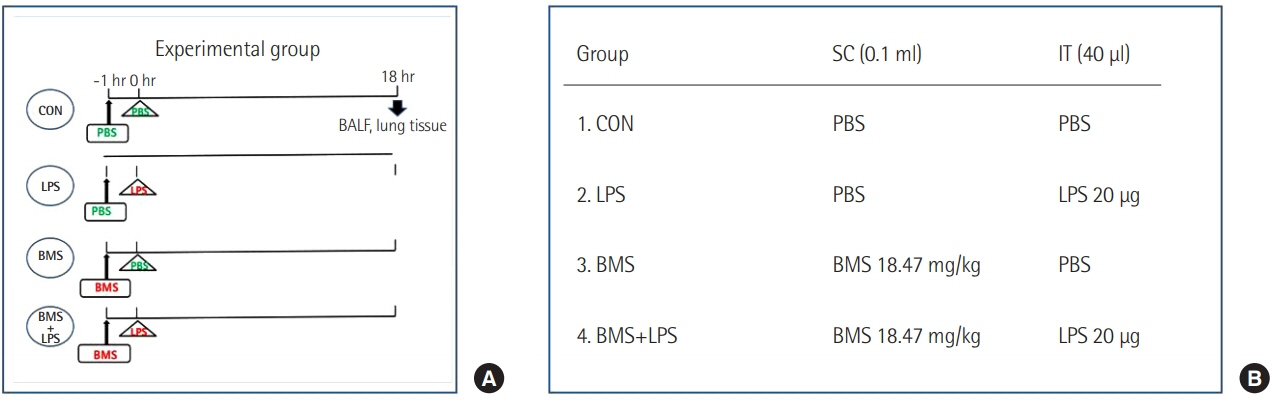
Mice received a subcutaneous injection of saline or BMS-470539 (18.47 mg/kg) 1 hour before an intratracheal injection of saline or lipopolysaccharide (20 µg). Mice were sacrificed to analyze the severity of pulmonary edema (lung wet-to-dry weight [W/D] ratio) and inflammatory responses (level of leukocytes, polymorphonuclear neutrophils [PMNs] and tumor necrosis factor alpha [TNF-α] in bronchoalveolar lavage fluid [BALF]), and neutrophil infiltration (myeloperoxidase activity). TNF-α activation was also measured in neutrophils from bone marrow. Survival was investigated in a second-hit sepsis mouse model.
RESULTS
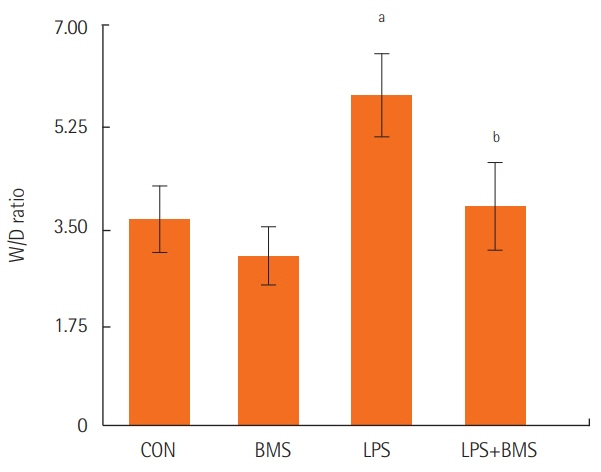
BMS-470539 improved sepsis-induced pulmonary edema, as demonstrated by a decreased W/D ratio (5.76%±0.83% to 3.81%±0.86%, P<0.05). The inflammatory response also improved, as shown by decreased levels of leukocytes (551±116 to 357±86×10²/mm³, P<0.05), PMNs (51.52%±16.23% to 18.41%±7.25%, P<0.01), and TNF-α (550±338 to 128±52 pg/ml, P<0.01) in the BALF. BMS-470539 also improved the inflammatory response, as shown by TNF-α levels (850±158 to 423±59 pg/ml, P<0.01) in neutrophils. BMS-470539 downregulated neutrophil infiltration in the lung (myeloperoxidase: 654±98 to 218±89 U/g, P<0.001). Lastly, BMS improved the survival rate (0% to 70%, P<0.01) in a mice multiple organ failure model.
CONCLUSIONS
BMS-470539 improved lipopolysaccharide-induced acute lung injury and mortality in mice by affecting the inflammatory response.
MeSH Terms
-
Acute Lung Injury*
Animals
Bone Marrow
Bronchoalveolar Lavage Fluid
Cytokines
Injections, Subcutaneous
Leukocytes
Lipopolysaccharides
Lung
Macrophages
Mice
Mortality
Multiple Organ Failure
Neutrophil Infiltration
Neutrophils
Pulmonary Edema
Receptor, Melanocortin, Type 1
Sepsis
Survival Rate
Tumor Necrosis Factor-alpha
Cytokines
Lipopolysaccharides
Receptor, Melanocortin, Type 1
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007; 369:1553–64.
Article2. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016; 315:788–800.
Article3. Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J. 2011; 87:612–22.
Article4. Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011; 17:293–307.
Article5. Chopra M, Reuben JS, Sharma AC. Acute lung injury: apoptosis and signaling mechanisms. Exp Biol Med (Maywood). 2009; 234:361–71.6. Wikberg JE, Muceniece R, Mandrika I, Prusis P, Lindblom J, Post C, et al. New aspects on the melanocortins and their receptors. Pharmacol Res. 2000; 42:393–420.
Article7. Kang L, McIntyre KW, Gillooly KM, Yang Y, Haycock J, Roberts S, et al. A selective small molecule agonist of the melanocortin-1 receptor inhibits lipopolysaccharide-induced cytokine accumulation and leukocyte infiltration in mice. J Leukoc Biol. 2006; 80:897–904.
Article8. Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003; 284:E468–74.
Article9. Maaser C, Kannengiesser K, Specht C, Lügering A, Brzoska T, Luger TA, et al. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. 2006; 55:1415–22.
Article10. Leoni G, Voisin MB, Carlson K, Getting S, Nourshargh S, Perretti M. The melanocortin MC(1) receptor agonist BMS-470539 inhibits leucocyte trafficking in the inflamed vasculature. Br J Pharmacol. 2010; 160:171–80.
Article11. Chen W, Li J, Qu H, Song Z, Yang Z, Huo J, et al. The melanocortin 1 receptor (MC1R) inhibits the inflammatory response in Raw 264.7 cells and atopic dermatitis (AD) mouse model. Mol Biol Rep. 2013; 40:1987–96.
Article12. Holloway PM, Durrenberger PF, Trutschl M, Cvek U, Cooper D, Orr AW, et al. Both MC1 and MC3 receptors provide protection from cerebral ischemia-reperfusion-induced neutrophil recruitment. Arterioscler Thromb Vasc Biol. 2015; 35:1936–44.13. Mykicki N, Herrmann AM, Schwab N, Deenen R, Sparwasser T, Limmer A, et al. Melanocortin-1 receptor activation is neuroprotective in mouse models of neuroinflammatory disease. Sci Transl Med. 2016; 8:362ra146.
Article14. Qiang X, Liotta AS, Shiloach J, Gutierrez JC, Wang H, Ochani M, et al. New melanocortin-like peptide of E. coli can suppress inflammation via the mammalian melanocortin-1 receptor (MC1R): possible endocrine-like function for microbes of the gut. NPJ Biofilms Microbiomes. 2017; 3:31.
Article15. Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccharide-induced neutrophilic inflammation in the lungs differs with age. Exp Lung Res. 2007; 33:375–84.16. Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, et al. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med. 2001; 163:762–9.
Article17. Ignar DM, Andrews JL, Jansen M, Eilert MM, Pink HM, Lin P, et al. Regulation of TNF-alpha secretion by a specific melanocortin-1 receptor peptide agonist. Peptides. 2003; 24:709–16.18. Montero-Melendez T, Gobbetti T, Cooray SN, Jonassen TE, Perretti M. Biased agonism as a novel strategy to harness the proresolving properties of melanocortin receptors without eliciting melanogenic effects. J Immunol. 2015; 194:3381–8.
Article19. Szarka RJ, Wang N, Gordon L, Nation PN, Smith RH. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. J Immunol Methods. 1997; 202:49–57.
Article20. Patel BV, Wilson MR, O’Dea KP, Takata M. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol. 2013; 190:4274–82.
Article21. Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 1995; 92:8016–20.
Article22. Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 1996; 17:675–9.23. Gonindard C, Goigoux C, Hollande E, D’Hinterland LD. The administration of an alpha-MSH analogue reduces the serum release of IL-1 alpha and TNF alpha induced by the injection of a sublethal dose of lipopolysaccharides in the BALB/c mouse. Pigment Cell Res. 1996; 9:148–53.24. Bhardwaj RS, Schwarz A, Becher E, Mahnke K, Aragane Y, Schwarz T, et al. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. J Immunol. 1996; 156:2517–21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of BMS-470539 on lipopolysaccharide-induced neutrophil activation
- Inhibitory effects of fenbendazole, an anthelmintics, on lipopolysaccharide-activated mouse bone marrow cells
- Diosmetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury through Activating the Nrf2 Pathway and Inhibiting the NLRP3 Inflammasome
- Ventilator-Induced Lung Injury
- Aspirin Reduces Acute Lung Injury in Rats Subjected to Severe Hemorrhage