Korean J Physiol Pharmacol.
2019 May;23(3):203-217. 10.4196/kjpp.2019.23.3.203.
Effects of heme oxygenase-1 upregulation on isoproterenol-induced myocardial infarction
- Affiliations
-
- 1Department of Physiology, Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt. menhag@mans.edu.eg
- 2Medical Experimental Research Center, Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt.
- 3Department of Medical Biochemistry, Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt.
- KMID: 2443608
- DOI: http://doi.org/10.4196/kjpp.2019.23.3.203
Abstract
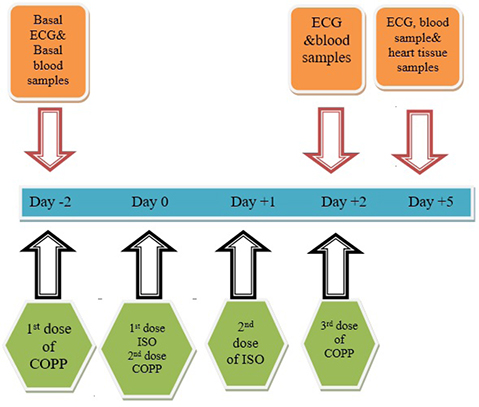
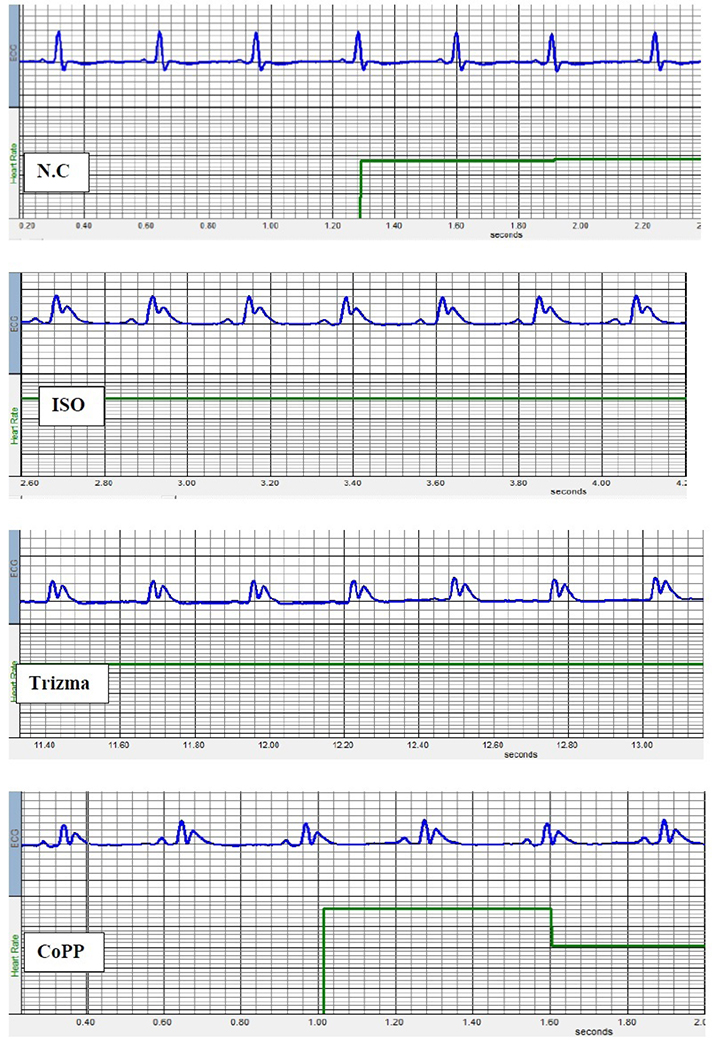
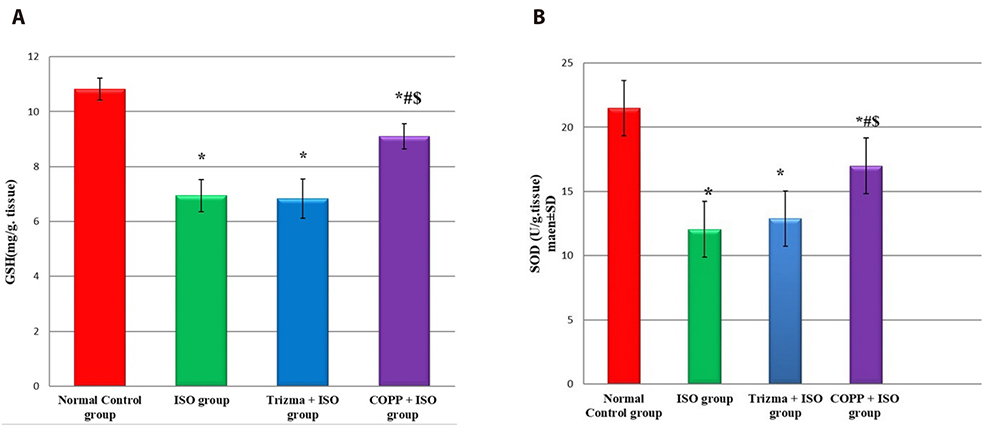
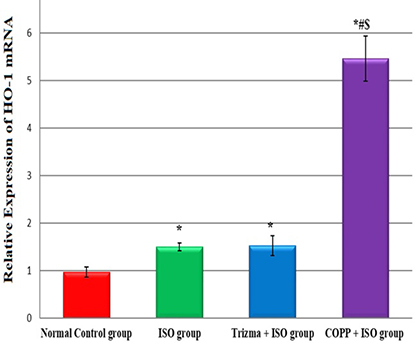
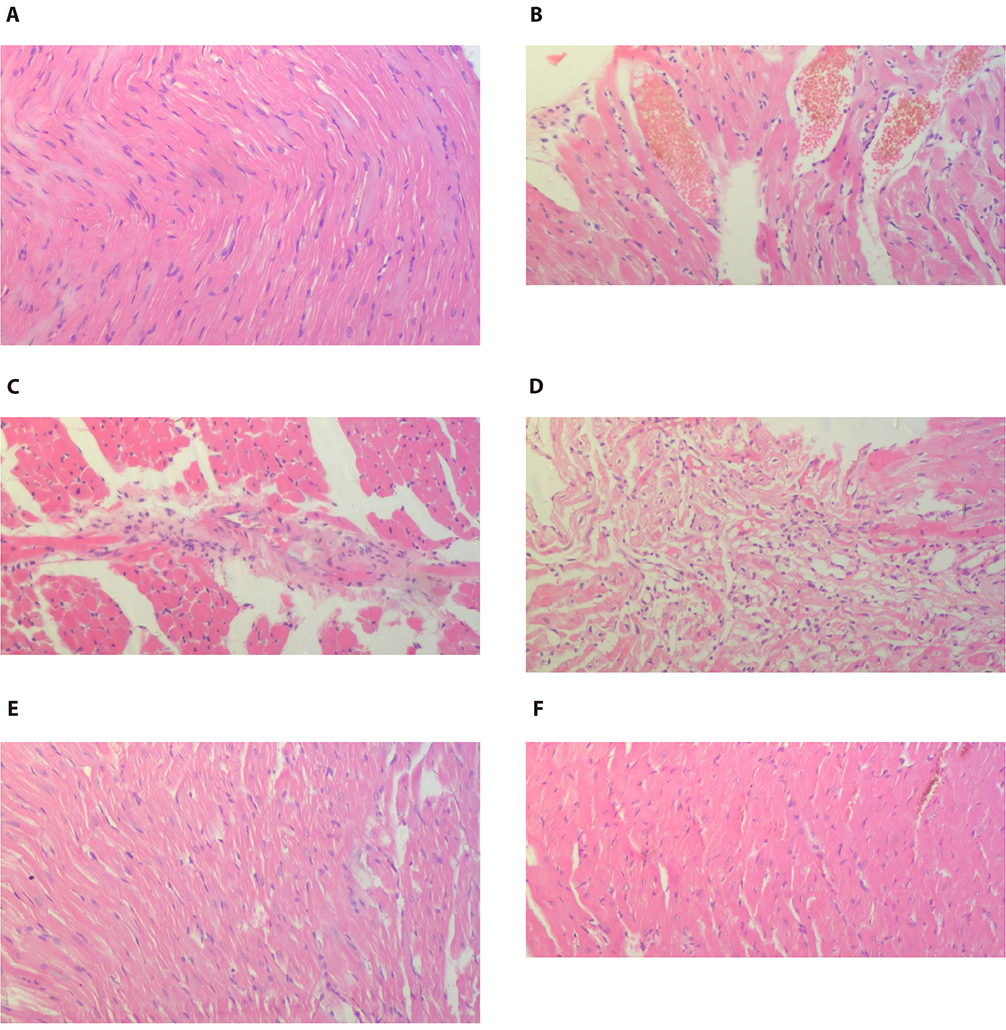
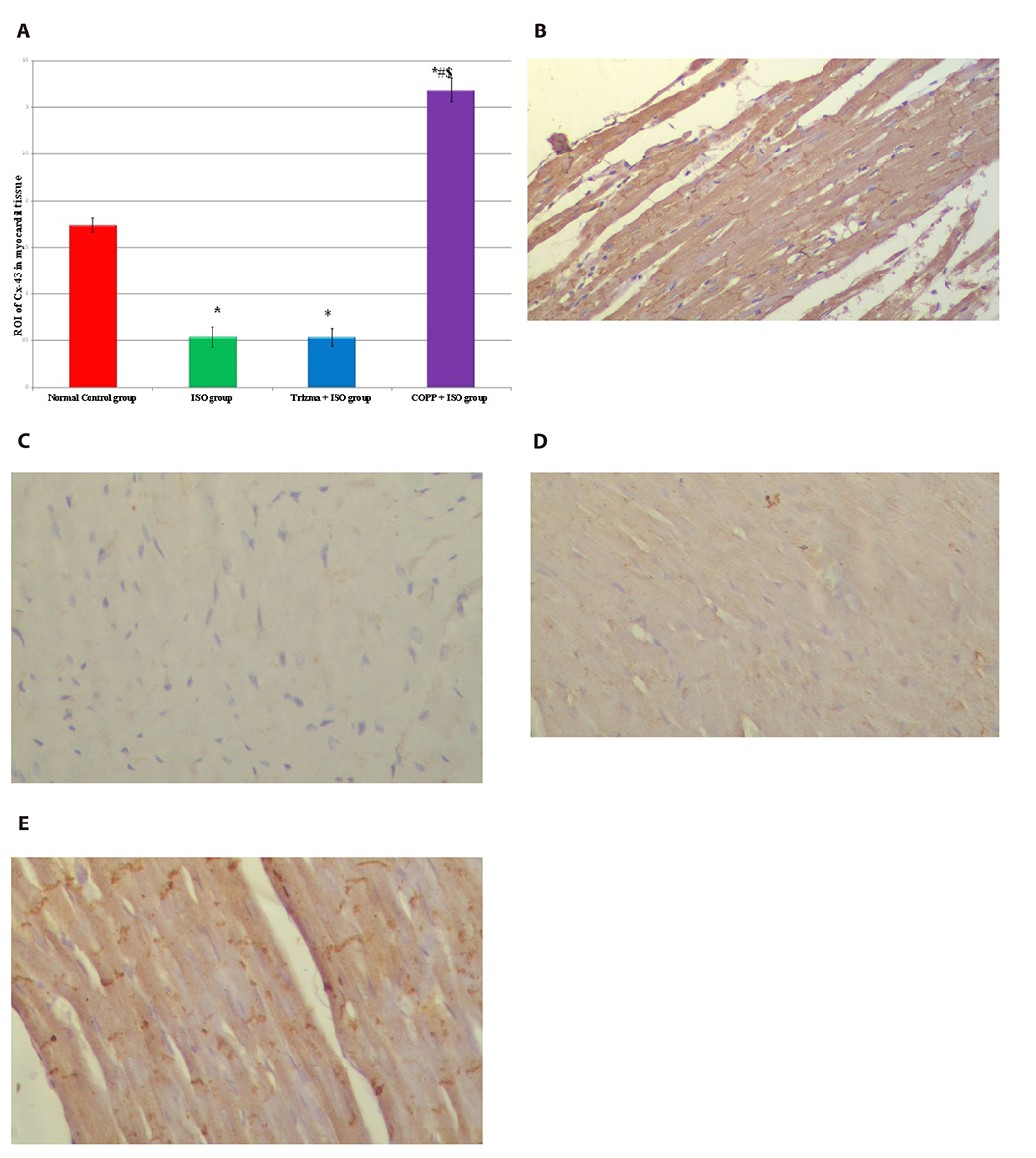
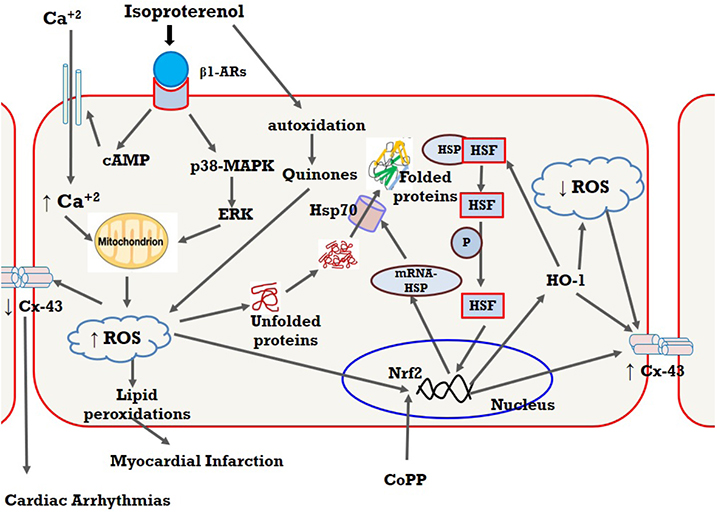
- The present study was designed to examine the effect of heme oxygenase-1 (HO-1) induction by cobalt protoporphyrin (CoPP) on the cardiac functions and morphology, electrocardiogram (ECG) changes, myocardial antioxidants (superoxide dismutase [SOD] and glutathione [GSH]), and expression of heat shock protein (Hsp) 70 and connexin 43 (Cx-43) in myocardial muscles in isoproterenol (ISO) induced myocardial infarction (MI). Thirty two adult male Sprague Dawely rats were divided into 4 groups (each 8 rats): normal control (NC) group, ISO group: received ISO at dose of 150 mg/kg body weight intraperitoneally (i.p.) for 2 successive days; ISO + Trizma group: received (ISO) and Trizma (solvent of CoPP) at dose of 5 mg/kg i.p. injection 2 days before injection of ISO, with ISO at day 0 and at day 2 after ISO injections; and ISO + CoPP group: received ISO and CoPP at a dose of 5 mg/kg dissolved in Trizma i.p. injection as Trizma. We found that, administration of ISO caused significant increase in heart rate, corrected QT interval, ST segment, cardiac enzymes (lactate dehydrogenase, creatine kinase-muscle/brain), cardiac HO-1, Hsp70 with significant attenuation in myocardial GSH, SOD, and Cx-43. On the other hand, administration of CoPP caused significant improvement in ECG parameters, cardiac enzymes, cardiac morphology; antioxidants induced by ISO with significant increase in HO-1, Cx-43, and Hsp70 expression in myocardium. In conclusions, we concluded that induction of HO-1 by CoPP ameliorates ISO-induced myocardial injury, which might be due to up-regulation of Hsp70 and gap junction protein (Cx-43).
Keyword
MeSH Terms
-
Adult
Animals
Antioxidants
Body Weight
Cobalt
Connexin 43
Connexins
Creatine
Electrocardiography
Glutathione
Hand
Heart Rate
Heat-Shock Proteins
Heme Oxygenase-1*
Heme*
HSP70 Heat-Shock Proteins
Humans
Isoproterenol
Male
Muscles
Myocardial Infarction*
Myocardium
Oxidoreductases
Rats
Tromethamine
Up-Regulation*
Antioxidants
Cobalt
Connexin 43
Connexins
Creatine
Glutathione
HSP70 Heat-Shock Proteins
Heat-Shock Proteins
Heme
Heme Oxygenase-1
Isoproterenol
Oxidoreductases
Tromethamine
Figure
Reference
-
1. Yusuf S, Ounpuu S. Tackling the growing global burden of atherosclerotic cardiovascular diseases. Eur J Cardiovasc Prev Rehabil. 2003; 10:236–239.
Article2. Hashmi S, Al-Salam S. Acute myocardial infarction and myocardial ischemia-reperfusion injury: a comparison. Int J Clin Exp Pathol. 2015; 8:8786–8796.3. Sathish V, Ebenezar KK, Devaki T. Synergistic effect of Nicorandil and Amlodipine on tissue defense system during experimental myocardial infarction in rats. Mol Cell Biochem. 2003; 243:133–138.4. Mahammad Rahmathulla SB, Kodidala LD. Origination and development of isoproterenol-induced myocardial infarction in male wistar rats. Int Res J Pharm. 2013; 4:26–35.5. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988; 2:2557–2568.
Article6. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006; 86:583–650.
Article7. Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001; 89:168–173.
Article8. Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, Griese DP, Dell'Acqua G, Mann MJ, Oyama J, Yet SF, Layne MD, Perrella MA, Dzau VJ. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002; 105:602–607.
Article9. Lakkisto P, Kytö V, Forsten H, Siren JM, Segersvärd H, Voipio-Pulkki LM, Laine M, Pulkki K, Tikkanen I. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur J Pharmacol. 2010; 635:156–164.10. Kappas A, Drummond GS. Control of heme metabolism with synthetic metalloporphyrins. J Clin Invest. 1986; 77:335–339.
Article11. Kusmic C, Barsanti C, Matteucci M, Vesentini N, Pelosi G, Abraham NG, L'Abbate A. Up-regulation of heme oxygenase-1 after infarct initiation reduces mortality, infarct size and left ventricular remodeling: experimental evidence and proof of concept. J Transl Med. 2014; 12:89.
Article12. Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci U S A. 2007; 104:20512–20516.
Article13. Bernardo BC, Sapra G, Patterson NL, Cemerlang N, Kiriazis H, Ueyama T, Febbraio MA, McMullen JR. Long-term overexpression of Hsp70 does not protect against cardiac dysfunction and adverse remodeling in a MURC transgenic mouse model with chronic heart failure and atrial fibrillation. PLoS One. 2015; 10:e0145173.
Article14. Barakat M, Gabr MM, Zhran F, El-Adl M, Hussein AM, Barakat N, Eldemerdash R. Upregulation of heme oxygenase 1 (HO-1) attenuates kidney damage, oxidative stress and inflammatory reaction during renal ischemia/ reperfusion injury. Gen Physiol Biophys. 2018; 37:193–204.15. Lakkisto P, Siren JM, Kytö V, Forsten H, Laine M, Pulkki K, Tikkanen I. Heme oxygenase-1 induction protects the heart and modulates cellular and extracellular remodelling after myocardial infarction in rats. Exp Biol Med (Maywood). 2011; 236:1437–1448.
Article16. Issan Y, Kornowski R, Aravot D, Shainberg A, Laniado-Schwartzman M, Sodhi K, Abraham NG, Hochhauser E. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS One. 2014; 9:e92246.
Article17. Metias EF, Aboelmaaty NM, Hussein AM, Abdallah EW, Abdelaziz A. Modulation of ECG, myocardial oxidative stress markers and connexion 43 expression by ascorbic acid and ferulic acid in isoproterenol-induced myocardial infarction in rats. Biochem Physiol. 2016; 5:210.
Article18. El-Wakf AM, El-Habibi ESM, Barakat NM, Attia AM, Hussein AM, Ali II. Cardiovascular toxic effects of chlorpyrifos: a possible protective role for pomegranate extracts. J Clin Toxicol. 2018; 8:374.19. Hu X, Fu W, Jiang H. HMGB1: a potential therapeutic target for myocardial ischemia and reperfusion injury. Int J Cardiol. 2012; 155:489.
Article20. Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003; 24:449–455.
Article21. Sun J, Kim SJ, Park MK, Kim HJ, Tsoy I, Kang YJ, Lee YS, Seo HG, Lee JH, Chang KC. Selective activation of adrenergic beta1 receptors induces heme oxygenase 1 production in RAW264.7 cells. FEBS Lett. 2005; 579:5494–5500.22. Ha YM, Ham SA, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Park MK, Chang KC. β1-adrenergic receptor-mediated HO-1 induction, via PI3K and p38 MAPK, by isoproterenol in RAW 264.7 cells leads to inhibition of HMGB1 release in LPS-activated RAW 264.7 cells and increases in survival rate of CLP-induced septic mice. Biochem Pharmacol. 2011; 82:769–777.23. Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens. 2014; 23:17–24.
Article24. Teodorescu C, Reinier K, Uy-Evanado A, Navarro J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm. 2011; 8:1562–1567.
Article25. Thippeswamy BS, Thakker SP, Tubachi S, Kalyani GA, Netra MK, Patil U, Desai S, Gavimath CC, Veerapur VP. Cardioprotective effect of Cucumis trigonus Roxb on isoproterenol-induced myocardial infarction in rat. Am J Pharmacol Toxicol. 2009; 4:29–37.26. Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010; 61:269–280.
Article27. El-Shitany NA, El-Desoky K. Protective effects of carvedilol and vitamin C against azithromycin-induced cardiotoxicity in rats via decreasing ROS, IL1-β, and TNF-α production and inhibiting NF-κB and caspase-3 expression. Oxid Med Cell Longev. 2016; 2016:1874762.28. Coppola G, Carità P, Corrado E, Borrelli A, Rotolo A, Guglielmo M, Nugara C, Ajello L, Santomauro M, Novo S. ST segment elevations: always a marker of acute myocardial infarction. Indian Heart J. 2013; 65:412–423.
Article29. Pósa A, Szabó R, Csonka A, Veszelka M, Berkó AM, Baráth Z, Ménesi R, Pávó I, Gyöngyösi M, László F, Kupai K, Varga C. Endogenous estrogen-mediated heme oxygenase regulation in experimental menopause. Oxid Med Cell Longev. 2015; 2015:429713.
Article30. Ndisang J. Heme oxygenase ameliorates electrocardiographic and hemodynamic parameters by potentiating insulin signaling in diabetic cardiomyopathy. Can J Cardiol. 2017; 33:S173–S174.
Article31. Khalil I, Ahmmed I, Ahmed R, Tanvir EM, Afroz R, Paul S, Gan SH, Alam N. Amelioration of isoproterenol-induced oxidative damage in rat myocardium by Withania somnifera leaf extract. Biomed Res Int. 2015; 2015:624159.32. French D, Wu A. Cardiac Markers. In : Wild D, editor. The immunoassay handbook. Jordan Hill: Elsevier Science;2013. p. 817–831.33. Bodor GS. Biochemical markers of myocardial damage. EJIFCC. 2016; 27:95–111.34. Panda VS, Naik SR. Cardioprotective activity of Ginkgo biloba Phytosomes in isoproterenol-induced myocardial necrosis in rats: a biochemical and histoarchitectural evaluation. Exp Toxicol Pathol. 2008; 60:397–404.
Article35. Zhou R, Xu Q, Zheng P, Yan L, Zheng J, Dai G. Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur J Pharmacol. 2008; 586:244–250.
Article36. Liu YT, Jia HM, Chang X, Ding G, Zhang HW, Zou ZM. The metabolic disturbances of isoproterenol induced myocardial infarction in rats based on a tissue targeted metabonomics. Mol Biosyst. 2013; 9:2823–2834.
Article37. Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985; 17:291–306.
Article38. Rajadurai M, Stanely Mainzen Prince P. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology. 2007; 230:178–188.
Article39. Woo J, Iyer S, Cornejo MC, Mori N, Gao L, Sipos I, Maines M, Buelow R. Stress protein-induced immunosuppression: inhibition of cellular immune effector functions following overexpression of haem oxygenase (HSP 32). Transpl Immunol. 1998; 6:84–93.
Article40. Katori M, Buelow R, Ke B, Ma J, Coito AJ, Iyer S, Southard D, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002; 73:287–292.41. Fang J, Qin H, Seki T, Nakamura H, Tsukigawa K, Shin T, Maeda H. Therapeutic potential of pegylated hemin for reactive oxygen species-related diseases via induction of heme oxygenase-1: results from a rat hepatic ischemia/reperfusion injury model. J Pharmacol Exp Ther. 2011; 339:779–789.
Article42. Ferenbach DA, Kluth DC, Hughes J. Hemeoxygenase-1 and renal ischaemia-reperfusion injury. Nephron Exp Nephrol. 2010; 115:e33–e37.
Article43. Nirmala C. Isoproterenol-induced myocardial infarction in rats: functional and biochemical alterations. Med Sci Res. 1994; 22:575–577.44. Díaz-Muñoz M, Alvarez-Pérez MA, Yáñez L, Vidrio S, Martínez L, Rosas G, Yáñez M, Ramírez S, de Sánchez VC. Correlation between oxidative stress and alteration of intracellular calcium handling in isoproterenol-induced myocardial infarction. Mol Cell Biochem. 2006; 289:125–136.
Article45. Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012; 2012:217037.
Article46. Svobodová A, Zdarilová A, Walterová D, Vostálová J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J Dermatol Sci. 2007; 48:213–224.
Article47. Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006; 20:2651–2653.
Article48. Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009; 47:1304–1309.
Article49. van Veen AA, van Rijen HV, Opthof T. Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc Res. 2001; 51:217–229.
Article50. Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kléber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000; 87:656–662.
Article51. Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008; 80:9–19.
Article52. Huang XD, Sandusky GE, Zipes DP. Heterogeneous loss of connexin43 protein in ischemic dog hearts. J Cardiovasc Electrophysiol. 1999; 10:79–91.
Article53. Sun JM, Wang CM, Guo Z, Hao YY, Xie YJ, Gu J, Wang AL. Reduction of isoproterenol-induced cardiac hypertrophy and modulation of myocardial connexin43 by a KATP channel agonist. Mol Med Rep. 2015; 11:1845–1850.
Article54. Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 2003; 17:1355–1357.
Article55. Calabrese V, Davinelli S, Luca M, Zella D, Calabrese EJ, Scapagnini G. Inflammaging, oxidative stress and carnosine: role of hormetic vitagenes. In : Preedy VR, editor. Imidazole dipeptides: chemistry, analysis, function and effects. Cambridge: Royal Society of Chemistry;2015. p. 238–256.56. Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002; 105:685–690.57. Li Z, Song Y, Xing R, Yu H, Zhang Y, Li Z, Gao W. Heat shock protein 70 acts as a potential biomarker for early diagnosis of heart failure. PLoS One. 2013; 8:e67964.
Article58. Tanwar V, Sachdeva J, Golechha M, Kumari S, Arya DS. Curcumin protects rat myocardium against isoproterenol-induced ischemic injury: attenuation of ventricular dysfunction through increased expression of Hsp27 along with strengthening antioxidant defense system. J Cardiovasc Pharmacol. 2010; 55:377–384.59. Tanonaka K, Toga W, Takeo S. [Induction of heat shock protein 70 in failing heart]. Nihon Yakurigaku Zasshi. 2004; 123:71–76.
Article60. Lakkisto P, Csonka C, Fodor G, Bencsik P, Voipio-Pulkki LM, Ferdinandy P, Pulkki K. The heme oxygenase inducer hemin protects against cardiac dysfunction and ventricular fibrillation in ischaemic/ reperfused rat hearts: role of connexin 43. Scand J Clin Lab Invest. 2009; 69:209–218.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- Heme Oxygenase-1 Induced by Aprotinin Inhibits Vascular Smooth Muscle Cell Proliferation Through Cell Cycle Arrest in Hypertensive Rats
- Effects of Heme Oxygenase-1 Expression in Mycophenolic Acid Induced Apoptosis of Jurkat Cell Lines
- Change of Expression and Activity of Heme Oxygenase-1 in Rat Corpus Cavernosum during Low-flow Priapism
- Effects of Oxidative Stress and Antioxidant on the Expression of Heme Oxygenase-1 in Human RPE