Ann Dermatol.
2012 May;24(2):189-193.
Effect of Probiotics on the Treatment of Children with Atopic Dermatitis
- Affiliations
-
- 1Department of Dermatology, Harran University Faculty of Medicine, Sanliurfa, Turkey. yavuzyesilova@gmail.com
- 2Department of Dermatology, Yuzuncu Yil University, Faculty of Medicine, Van, Turkey.
- 3Department of Microbiology, Yuzuncu Yil University, Faculty of Medicine, Van, Turkey.
Abstract
- BACKGROUND
Atopic dermatitis, a chronic recurrent disease, is frequently encountered in clinical practice. In the last 30 years, the prevalence of atopic dermatitis has rapidly increased due to industrialization. Therefore, there have been attempts in recent years to find new ways of treating and preventing atopic dermatitis.
OBJECTIVE
In this double-blind, randomized, placebo-controlled study, a combination of Bifidobacterium bifidum, Lactobacillus acidophilus, Lactobacillus casei, and Lactobacillus salivarius strains were evaluated in the treatment of atopic dermatitis in pediatric patients.
METHODS
Forty pediatric patients (23 males and 17 females) aged 1~13 years were enrolled. One eligible individual who was approached declined to participate. The probiotic group was administered a probiotic complex containing B. bifidum, L. acidophilus, L. casei, and L. salivarius for 8 weeks. The placebo group, on the other hand, was administered skim milk powder and dextrose. All of the parameters including serum cytokines, eosinophil cationic protein), SCORing Atopic Dermatitis (SCORAD) index, and total serum immunoglobulin E (IgE) were measured in both the probiotic group and the placebo group at the end of 8 weeks.
RESULTS
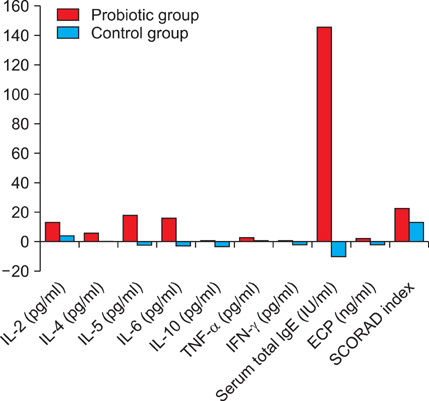
Probiotic intervention in pediatric atopic dermatitis patients effectively reduced the SCORAD index and serum cytokines interleukin (IL)-5, IL-6, interferon (IFN)-gamma, and total serum IgE levels, but did not reduce levels of serum cytokines IL-2, IL-4, IL-10, ECP, or tumor necrosis factor-alpha (TNF-alpha) compared to the placebo group.
CONCLUSION
Our study found probiotics to be effective in reducing atopic dermatitis patients' SCORAD index, serum IL-5, IL-6, IFN-gamma, and total serum IgE levels but not effective in reducing serum IL-2, IL-4, IL-10, ECP, or TNF-alpha levels.
Keyword
MeSH Terms
-
Aged
Bifidobacterium
Child
Cytokines
Dermatitis, Atopic
Eosinophils
Glucose
Hand
Humans
Immunoglobulin E
Immunoglobulins
Interferons
Interleukin-10
Interleukin-2
Interleukin-4
Interleukin-5
Interleukin-6
Interleukins
Lactobacillus
Lactobacillus acidophilus
Lactobacillus casei
Male
Milk
Prevalence
Probiotics
Tumor Necrosis Factor-alpha
Cytokines
Glucose
Immunoglobulin E
Immunoglobulins
Interferons
Interleukin-10
Interleukin-2
Interleukin-4
Interleukin-5
Interleukin-6
Interleukins
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Kristal L, Klein PA. Atopic dermatitis in infants and children. An update. Pediatr Clin North Am. 2000. 47:877–895.2. Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001. 56:813–824.
Article3. Bengmark S, García de Lorenzo A, Culebras JM. Use of pro-, pre- and synbiotics in the ICU--future options. Nutr Hosp. 2001. 16:239–256.4. Gill HS, Guarner F. Probiotics and human health: a clinical perspective. Postgrad Med J. 2004. 80:516–526.
Article5. Cosickic A, Skokic F, Colic-Hadzic B, Jahic M. Clinical characteristics and estimation severity of the atopic dermatitis in children. Med Arh. 2010. 64:178–182.6. Carr WW. Improvements in skin-testing technique. Allergy Asthma Proc. 2006. 27:100–103.7. Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007. 157:645–648.
Article8. Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006. 83:1256–1264.
Article9. Servin AL, Coconnier MH. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol. 2003. 17:741–754.
Article10. Morita H, He F, Fuse T, Ouwehand AC, Hashimoto H, Hosoda M, et al. Adhesion of lactic acid bacteria to caco-2 cells and their effect on cytokine secretion. Microbiol Immunol. 2002. 46:293–297.
Article11. Maassen CB, van Holten-Neelen C, Balk F, den Bak-Glashouwer MJ, Leer RJ, Laman JD, et al. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine. 2000. 18:2613–2623.
Article12. Roessler A, Friedrich U, Vogelsang H, Bauer A, Kaatz M, Hipler UC, et al. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin Exp Allergy. 2008. 38:93–102.
Article13. Betsi GI, Papadavid E, Falagas ME. Probiotics for the treatment or prevention of atopic dermatitis: a review of the evidence from randomized controlled trials. Am J Clin Dermatol. 2008. 9:93–103.14. Weston S, Halbert A, Richmond P, Prescott SL. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005. 90:892–897.
Article15. Van Leent EJM, Bos JD. Katsambas AD, Lotti TM, editors. Atopic dermatitis. European handbook of dermatological treatments. 2003. 2nd ed. Berlin: Springer-Verlag;54–62.
Article16. Isolauri E. Probiotics in the prevention and treatment of allergic disease. Pediatr Allergy Immunol. 2001. 12:56–59.
Article17. Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001. 357:1076–1079.
Article18. Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003. 361:1869–1871.
Article19. Sistek D, Kelly R, Wickens K, Stanley T, Fitzharris P, Crane J. Is the effect of probiotics on atopic dermatitis confined to food sensitized children? Clin Exp Allergy. 2006. 36:629–633.
Article20. Brouwer ML, Wolt-Plompen SA, Dubois AE, van der Heide S, Jansen DF, Hoijer MA, et al. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo-controlled trial. Clin Exp Allergy. 2006. 36:899–906.
Article21. Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005. 60:494–500.
Article22. Prescott SL, Dunstan JA, Hale J, Breckler L, Lehmann H, Weston S, et al. Clinical effects of probiotics are associated with increased interferon-gamma responses in very young children with atopic dermatitis. Clin Exp Allergy. 2005. 35:1557–1564.
Article23. Ouwehand AC. Antiallergic effects of probiotics. J Nutr. 2007. 137:794S–797S.
Article24. Winkler P, Ghadimi D, Schrezenmeir J, Kraehenbuhl JP. Molecular and cellular basis of microflora-host interactions. J Nutr. 2007. 137:756S–772S.
Article25. Taylor AL, Hale J, Wiltschut J, Lehmann H, Dunstan JA, Prescott SL. Effects of probiotic supplementation for the first 6 months of life on allergen- and vaccine-specific immune responses. Clin Exp Allergy. 2006. 36:1227–1235.
Article26. Miniello VL, Brunetti L, Tesse R, Natile M, Armenio L, Francavilla R. Lactobacillus reuteri modulates cytokines production in exhaled breath condensate of children with atopic dermatitis. J Pediatr Gastroenterol Nutr. 2010. 50:573–576.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of probiotics on the prevention of atopic dermatitis
- Probiotics as an Immune Modulator for Allergic Disorders
- The Clinical Effect of Topical Diphenylcyclopropenone (DPCP) Therapy in the Treatment of Atopic Dermatitis in Children and Adolescents
- Microbiome Research in Atopic Dermatitis
- Update on management of pediatric atopic dermatitis