Ann Dermatol.
2012 May;24(2):115-125.
Role of Keratinocytes in the Development of Vitiligo
- Affiliations
-
- 1Department of Dermatology, Dongguk University Ilsan Hospital, Dongguk University Graduate School of Medicine, Goyang, Korea. lay5604@naver.com
Abstract
- Vitiligo is an acquired depigmentary disorder of the skin that results from the loss of functioning epidermal melanocytes. Most studies on vitiligo have concentrated on the abnormality of melanocytes rather than the abnormality of keratinocytes; however, epidermal melanocytes form a functional and structural unit with neighboring keratinocytes. In fact, direct cell-to cell contact stimulates in vitro proliferation of melanocytes, and growth factors produced by adjacent keratinocytes regulate the proliferation and differentiation of melanocytes. The potential role of keratinocyte-derived cytokines has also been presented. We focused on the structural changes in vitiliginous keratinocytes, which may result in loss of melanocytes, to examine the pathomechanism of vitiligo. The results of a comparison between depigmented and normally pigmented epidermis in patients with vitiligo showed that the keratinocytes in the depigmented epidermis were more vulnerable to apoptosis. Impaired Phosphatidylinositol 3-kinase (PI3K)/serine/threonine protein kinase (Akt) activation followed by reduced nuclear factor-kappaB activation under increased tumor necrosis factor-alpha levels was demonstrated as a mechanism for keratinocyte apoptosis. The role of aquaporin 3 in keratinocyte apoptosis was addressed based on the relationship between the PI3K/AKT pathway and the E-cadherin-catenin complex. Apoptotic keratinocytes induced a lower expression of keratinocyte-derived factors, including stem cell factor, in depigmented epidermis, resulting in passive melanocyte death.
Keyword
MeSH Terms
-
Apoptosis
Aquaporin 3
Cytokines
Epidermis
Humans
Intercellular Signaling Peptides and Proteins
Keratinocytes
Melanocytes
NF-kappa B
Phosphatidylinositol 3-Kinase
Protein Kinases
Skin
Stem Cell Factor
Tumor Necrosis Factor-alpha
Vitiligo
Aquaporin 3
Cytokines
Intercellular Signaling Peptides and Proteins
NF-kappa B
Phosphatidylinositol 3-Kinase
Protein Kinases
Stem Cell Factor
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Namazi MR. Neurogenic dysregulation, oxidative stress, autoimmunity, and melanocytorrhagy in vitiligo: can they be interconnected? Pigment Cell Res. 2007. 20:360–363.
Article2. Guerra L, Dellambra E, Brescia S, Raskovic D. Vitiligo: pathogenetic hypotheses and targets for current therapies. Curr Drug Metab. 2010. 11:451–467.
Article3. Sviderskaya EV, Wakeling WF, Bennett DC. A cloned, immortal line of murine melanoblasts inducible to differentiate to melanocytes. Development. 1995. 121:1547–1557.
Article4. Moellmann G, Klein-Angerer S, Scollay DA, Nordlund JJ, Lerner AB. Extracellular granular material and degeneration of keratinocytes in the normally pigmented epidermis of patients with vitiligo. J Invest Dermatol. 1982. 79:321–330.
Article5. Bhawan J, Bhutani LK. Keratinocyte damage in vitiligo. J Cutan Pathol. 1983. 10:207–212.
Article6. Yu HS, Kao CH, Yu CL. Coexistence and relationship of antikeratinocyte and antimelanocyte antibodies in patients with non-segmental-type vitiligo. J Invest Dermatol. 1993. 100:823–828.
Article7. Steinert PM, Idler WW, Zimmerman SB. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976. 108:547–567.
Article8. Bowden PE, Wood EJ, Cunliffe WJ. Comparison of prekeratin and keratin polypeptides in normal and psoriatic human epidermis. Biochim Biophys Acta. 1983. 743:172–179.
Article9. Skerrow D, Hunter I. Protein modifications during the keratinization of normal and psoriatic human epidermis. Biochim Biophys Acta. 1978. 537:474–484.
Article10. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995. 267:1456–1462.
Article11. Dive C, Gregory CD, Phipps DJ, Evans DL, Milner AE, Wyllie AH. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim Biophys Acta. 1992. 1133:275–285.
Article12. Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994. 84:1415–1420.
Article13. Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995. 182:1545–1556.
Article14. van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998. 31:1–9.
Article15. Sun XM, Snowden RT, Skilleter DN, Dinsdale D, Ormerod MG, Cohen GM. A flow-cytometric method for the separation and quantitation of normal and apoptotic thymocytes. Anal Biochem. 1992. 204:351–356.
Article16. Guchelaar HJ, Vermes I, Koopmans RP, Reutelingsperger CP, Haanen C. Apoptosis- and necrosis-inducing potential of cladribine, cytarabine, cisplatin, and 5-fluorouracil in vitro: a quantitative pharmacodynamic model. Cancer Chemother Pharmacol. 1998. 42:77–83.
Article17. Mangili F, Cigala C, Santambrogio G. Staining apoptosis in paraffin sections. Advantages and limits. Anal Quant Cytol Histol. 1999. 21:273–276.18. Lee AY, Youm YH, Kim NH, Yang H, Choi WI. Keratinocytes in the depigmented epidermis of vitiligo are more vulnerable to trauma (suction) than keratinocytes in the normally pigmented epidermis, resulting in their apoptosis. Br J Dermatol. 2004. 151:995–1003.
Article19. Moretti S, Fabbri P, Baroni G, Berti S, Bani D, Berti E, et al. Keratinocyte dysfunction in vitiligo epidermis: cytokine microenvironment and correlation to keratinocyte apoptosis. Histol Histopathol. 2009. 24:849–857.20. Stennicke HR, Salvesen GS. Caspases - controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta. 2000. 1477:299–306.
Article21. Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin Immunol. 2003. 15:185–193.
Article22. Weil M, Raff MC, Braga VM. Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr Biol. 1999. 9:361–364.
Article23. Bowen AR, Hanks AN, Allen SM, Alexander A, Diedrich MJ, Grossman D. Apoptosis regulators and responses in human melanocytic and keratinocytic cells. J Invest Dermatol. 2003. 120:48–55.
Article24. Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis - the p53 network. J Cell Sci. 2003. 116:4077–4085.
Article25. Harada H, Grant S. Apoptosis regulators. Rev Clin Exp Hematol. 2003. 7:117–138.26. Walczak H, Haas TL. Biochemical analysis of the native TRAIL death-inducing signaling complex. Methods Mol Biol. 2008. 414:221–239.
Article27. Dupré A, Christol B. Cockade-like vitiligo and linear vitiligo a variant of fitzpatrick's trichrome vitiligo. Arch Dermatol Res. 1978. 262:197–203.28. Gauthier Y, Cario-Andre M, Lepreux S, Pain C, Taïeb A. Melanocyte detachment after skin friction in non lesional skin of patients with generalized vitiligo. Br J Dermatol. 2003. 148:95–101.
Article29. Badri AM, Todd PM, Garioch JJ, Gudgeon JE, Stewart DG, Goudie RB. An immunohistological study of cutaneous lymphocytes in vitiligo. J Pathol. 1993. 170:149–155.
Article30. van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, Tigges B, Westerhof W, Das P. Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab Invest. 2000. 80:1299–1309.
Article31. Wańkowicz-Kalińska A, van den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, et al. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest. 2003. 83:683–695.
Article32. van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009. 129:2220–2232.
Article33. Moretti S, Spallanzani A, Amato L, Hautmann G, Gallerani I, Fabiani M, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res. 2002. 15:87–92.
Article34. Birol A, Kisa U, Kurtipek GS, Kara F, Kocak M, Erkek E, et al. Increased tumor necrosis factor alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the lesional skin of patients with nonsegmental vitiligo. Int J Dermatol. 2006. 45:992–993.
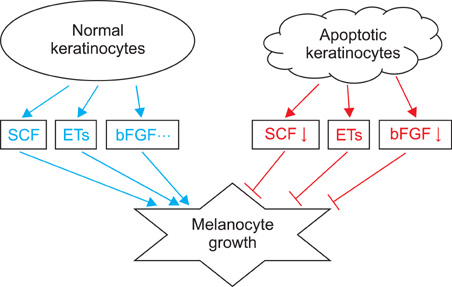
Article35. Gordon PR, Mansur CP, Gilchrest BA. Regulation of human melanocyte growth, dendricity, and melanization by keratinocyte derived factors. J Invest Dermatol. 1989. 92:565–572.
Article36. Rossi D, Gaidano G. Messengers of cell death: apoptotic signaling in health and disease. Haematologica. 2003. 88:212–218.37. Tartaglia LA, Pennica D, Goeddel DV. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem. 1993. 268:18542–18548.
Article38. Kim NH, Jeon S, Lee HJ, Lee AY. Impaired PI3K/Akt activation-mediated NF-kappaB inactivation under elevated TNF-alpha is more vulnerable to apoptosis in vitiliginous keratinocytes. J Invest Dermatol. 2007. 127:2612–2617.
Article39. Fulda S, Strauss G, Meyer E, Debatin KM. Functional CD95 ligand and CD95 death-inducing signaling complex in activation-induced cell death and doxorubicin-induced apoptosis in leukemic T cells. Blood. 2000. 95:301–308.
Article40. Lu B, Wang L, Stehlik C, Medan D, Huang C, Hu S, et al. Phosphatidylinositol 3-kinase/Akt positively regulates Fas (CD95)-mediated apoptosis in epidermal Cl41 cells. J Immunol. 2006. 176:6785–6793.
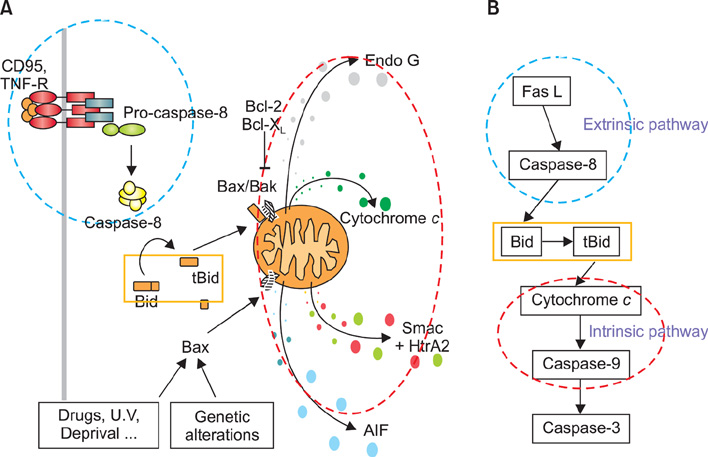
Article41. Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999. 274:1156–1163.
Article42. Singh R, Pervin S, Chaudhuri G. Caspase-8-mediated BID cleavage and release of mitochondrial cytochrome c during Nomega-hydroxy-L-arginine-induced apoptosis in MDA-MB-468 cells. Antagonistic effects of L-ornithine. J Biol Chem. 2002. 277:37630–37636.
Article43. Li T, Lu C, Xia Z, Xiao B, Luo Y. Inhibition of caspase-8 attenuates neuronal death induced by limbic seizures in a cytochrome c-dependent and Smac/DIABLO-independent way. Brain Res. 2006. 1098:204–211.
Article44. Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999. 401:82–85.
Article45. Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996. 84:299–308.
Article46. Wallach D, Boldin MP, Kovalenko AV, Malinin NL, Mett IL, Camonis JH. The yeast two-hybrid screening technique and its use in the study of protein-protein interactions in apoptosis. Curr Opin Immunol. 1998. 10:131–136.
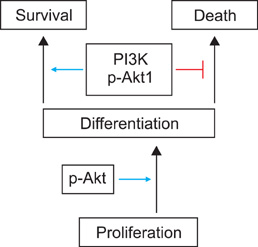
Article47. Thrash BR, Menges CW, Pierce RH, McCance DJ. AKT1 provides an essential survival signal required for differentiation and stratification of primary human keratinocytes. J Biol Chem. 2006. 281:12155–12162.
Article48. Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem. 2005. 280:32856–32865.
Article49. Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995. 376:337–341.
Article50. Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003. 17:1352–1365.
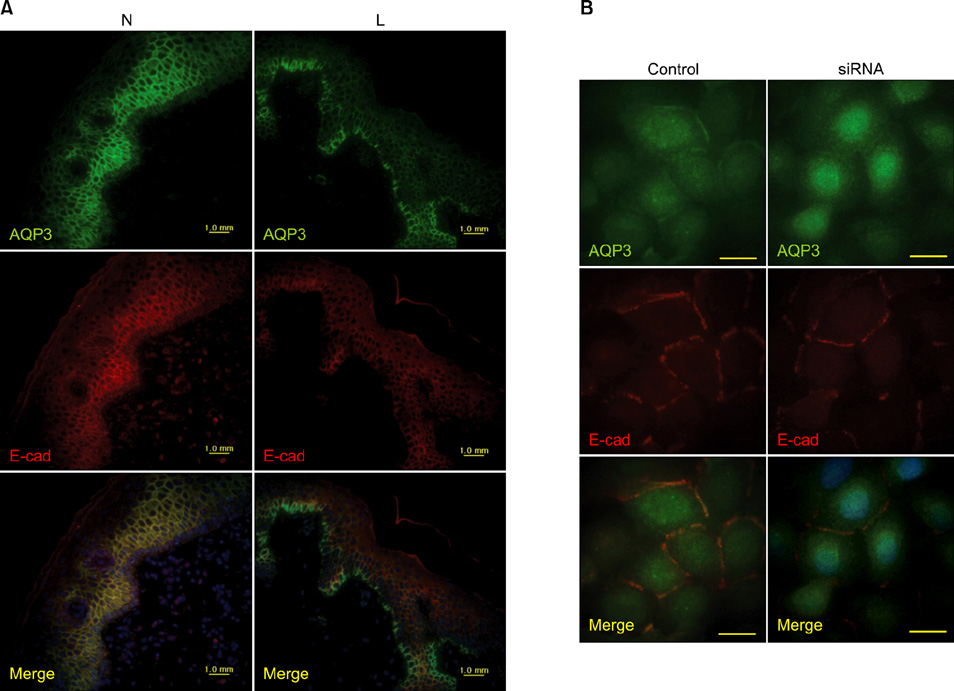
Article51. Kim NH, Lee AY. Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J Invest Dermatol. 2010. 130:2231–2239.
Article52. Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007. 282:8695–8703.
Article53. Janes SM, Ofstad TA, Campbell DH, Watt FM, Prowse DM. Transient activation of FOXN1 in keratinocytes induces a transcriptional programme that promotes terminal differentiation: contrasting roles of FOXN1 and Akt. J Cell Sci. 2004. 117:4157–4168.
Article54. Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002. 3:199–209.
Article55. Tinkle CL, Pasolli HA, Stokes N, Fuchs E. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci U S A. 2008. 105:15405–15410.
Article56. Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci U S A. 1995. 92:4328–4331.
Article57. Sougrat R, Morand M, Gondran C, Barré P, Gobin R, Bonté F, et al. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J Invest Dermatol. 2002. 118:678–685.
Article58. Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol. 2007. 178:323–335.
Article59. Gauthier Y, Cario-Andre M, Lepreux S, Pain C, Taïeb A. Melanocyte detachment after skin friction in non lesional skin of patients with generalized vitiligo. Br J Dermatol. 2003. 148:95–101.
Article60. Lee AY, Kim NH, Choi WI, Youm YH. Less keratinocyte-derived factors related to more keratinocyte apoptosis in depigmented than normally pigmented suction-blistered epidermis may cause passive melanocyte death in vitiligo. J Invest Dermatol. 2005. 124:976–983.
Article61. Hirobe T. Role of keratinocyte-derived factors involved in regulating the proliferation and differentiation of mammalian epidermal melanocytes. Pigment Cell Res. 2005. 18:2–12.
Article62. Morita E, Lee DG, Sugiyama M, Yamamoto S. Expression of c-kit ligand in human keratinocytes. Arch Dermatol Res. 1994. 286:273–277.
Article63. Hachiya A, Kobayashi A, Ohuchi A, Takema Y, Imokawa G. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001. 116:578–586.
Article64. Hirobe T, Osawa M, Nishikawa S. Steel factor controls the proliferation and differentiation of neonatal mouse epidermal melanocytes in culture. Pigment Cell Res. 2003. 16:644–655.
Article65. Kunisada T, Lu SZ, Yoshida H, Nishikawa S, Nishikawa S, Mizoguchi M, et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998. 187:1565–1573.
Article66. Longley BJ Jr, Morganroth GS, Tyrrell L, Ding TG, Anderson DM, Williams DE, et al. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N Engl J Med. 1993. 328:1302–1307.
Article67. Welker P, Grabbe J, Gibbs B, Zuberbier T, Henz BM. Human mast cells produce and differentially express both soluble and membrane-bound stem cell factor. Scand J Immunol. 1999. 49:495–500.
Article68. Jacobsen MD, Weil M, Raff MC. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996. 133:1041–1051.
Article69. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972. 26:239–257.
Article70. Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980. 68:251–306.
Article71. Ledda-Columbano GM, Columbano A, Coni P, Faa G, Pani P. Cell deletion by apoptosis during regression of renal hyperplasia. Am J Pathol. 1989. 135:657–662.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aquaporin-3 Downregulation in Vitiligo Keratinocytes Increases Oxidative Stress of Melanocytes
- Detection of Antibodies to Human Keratinocytes in Vitiligo by Western blotting
- Increased Sensitivity of Keratinocytes to Oxidative Stress in Vitiligo
- The Effects of Tacrolimus on Keratinocytes and Melanocytes of Vitiligo
- Clinical and Histopathological Characteristics of Trichrome Vitiligo