Allergy Asthma Immunol Res.
2019 Mar;11(2):267-279. 10.4168/aair.2019.11.2.267.
Intranasal Treatment With 1, 25-Dihydroxyvitamin D3 Alleviates Allergic Rhinitis Symptoms in a Mouse Model
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 2Center of Morphological Experiment, Medical College of Yanbian University, Yanji, China.
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. dongkim@snu.ac.kr
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
- KMID: 2431858
- DOI: http://doi.org/10.4168/aair.2019.11.2.267
Abstract
- PURPOSE
Vitamin D is a potent immunomodulator. However, its role in the pathogenesis of allergic rhinitis is unclear.
METHODS
The aim of this study was to evaluate the antiallergic effect of intranasally applied vitamin D in an allergic rhinitis mouse model. BALB/c mice were intraperitoneally sensitized with ovalbumin (OVA) and alum before they were intranasally challenged with OVA. Then, they were intranasally administered 1, 25-dihydroxyvitamin D3 (0.02 μg) or solvent. Allergic symptom scores, eosinophil infiltration, cytokine mRNA levels (interleukin [IL]-4, IL-5, IL-10, IL-13 and interferon-γ) in the nasal tissue, and serum total immunoglobulin E (IgE) and OVA-specific IgE, IgG1, and IgG2a were analyzed and compared with negative and positive control groups. Cervical lymph nodes (LNs) were harvested for flow cytometry analysis and cell proliferation assay.
RESULTS
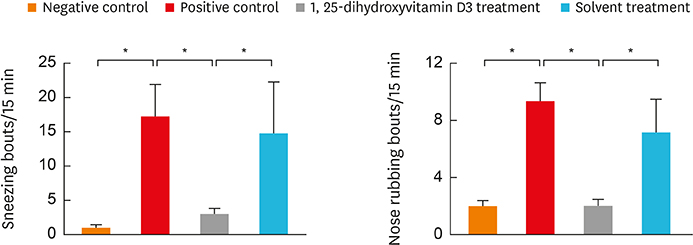
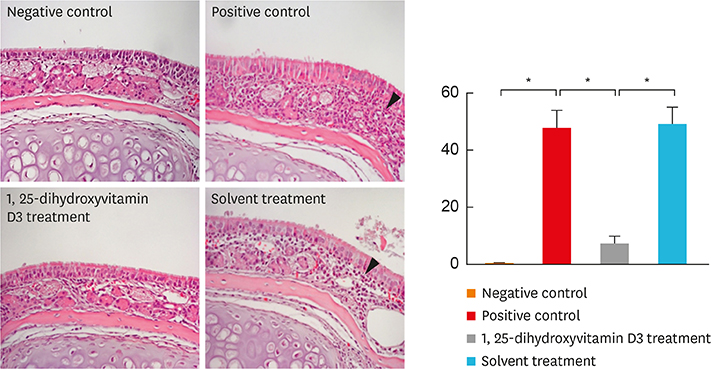
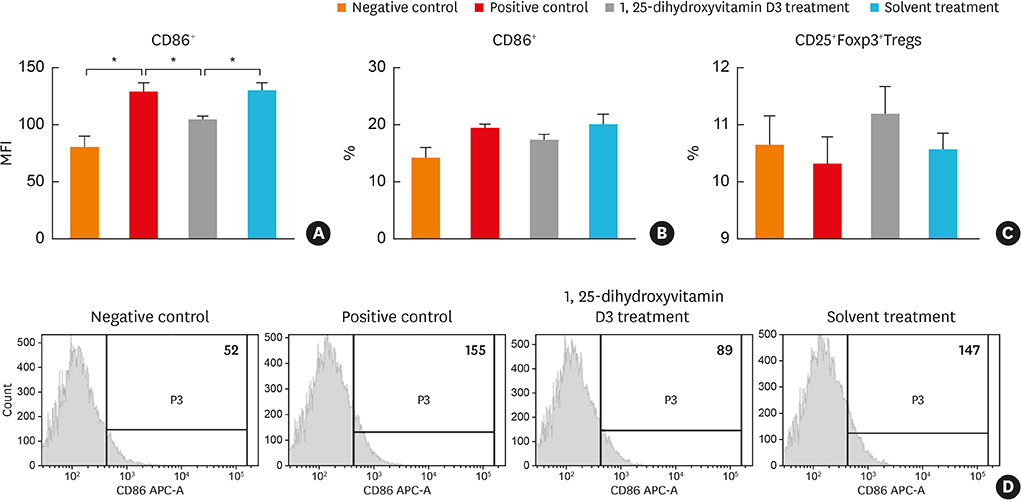
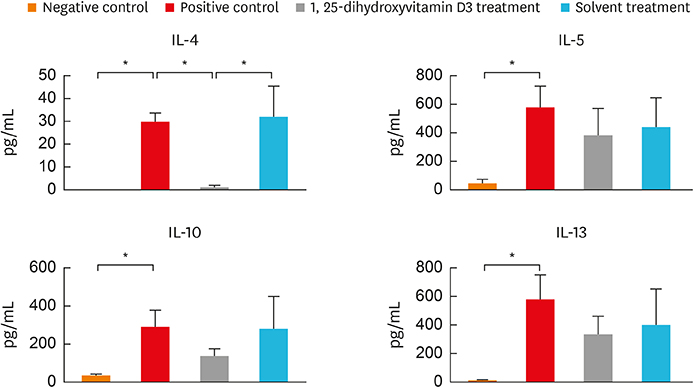
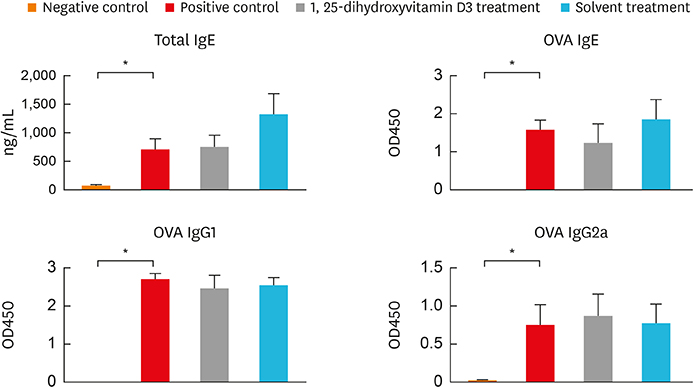
In the treatment group, allergic symptom scores, eosinophil infiltration, and mRNA levels of IL-4 and IL-13 were significantly lower in the nasal tissue than in the positive control group. The IL-5 mRNA level, serum total IgE, and OVA-specific IgE and IgG1 levels decreased in the treatment group; however, the difference was not significant. In the cervical LNs, CD86 expression had been down-regulated in CD11c+major histocompatibility complex II-high (MHCIIhigh) in the treatment group. Additionally, IL-4 secretion in the lymphocyte culture from cervical LNs significantly decreased.
CONCLUSIONS
The results confirm the antiallergic effect of intranasal 1,25-dihydroxyvitamin D3. It decreases CD 86 expression among CD11c+MHCIIhigh cells and T-helper type 2-mediated inflammation in the cervical LNs. Therefore, topically applied 1,25-dihydroxyvitamin D3 can be a future therapeutic agent for allergic rhinitis.
Keyword
MeSH Terms
-
Administration, Intranasal
Animals
Anti-Allergic Agents
Calcitriol
Cell Proliferation
Dendritic Cells
Eosinophils
Flow Cytometry
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Inflammation
Interleukin-10
Interleukin-13
Interleukin-4
Interleukin-5
Lymph Nodes
Lymphocytes
Major Histocompatibility Complex
Mice*
Models, Animal
Ovalbumin
Ovum
Rhinitis, Allergic*
RNA, Messenger
Vitamin D
Anti-Allergic Agents
Calcitriol
Immunoglobulin E
Immunoglobulin G
Immunoglobulins
Interleukin-10
Interleukin-13
Interleukin-4
Interleukin-5
Ovalbumin
RNA, Messenger
Vitamin D
Figure
Reference
-
1. Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009; 31:412–424.
Article2. Aryan Z, Rezaei N, Camargo CA Jr. Vitamin D status, aeroallergen sensitization, and allergic rhinitis: a systematic review and meta-analysis. Int Rev Immunol. 2017; 36:41–53.
Article3. Cheng HM, Kim S, Park GH, Chang SE, Bang S, Won CH, et al. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J Allergy Clin Immunol. 2014; 133:1048–1055.
Article4. Tian HQ, Cheng L. The role of vitamin D in allergic rhinitis. Asia Pac Allergy. 2017; 7:65–73.
Article5. Bartels LE, Hvas CL, Agnholt J, Dahlerup JF, Agger R. Human dendritic cell antigen presentation and chemotaxis are inhibited by intrinsic 25-hydroxy vitamin D activation. Int Immunopharmacol. 2010; 10:922–928.
Article6. Ferreira GB, van Etten E, Verstuyf A, Waer M, Overbergh L, Gysemans C, et al. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab Res Rev. 2011; 27:933–941.7. Hubo M, Trinschek B, Kryczanowsky F, Tuettenberg A, Steinbrink K, Jonuleit H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front Immunol. 2013; 4:82.
Article8. Percy DH, Barthold SW. Pathology of laboratory rodents and rabbits. 3rd ed. Ames, IA: Blackwell Publishing Professional;2007.9. Shklovskaya E, Roediger B, Fazekas de St Groth B. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo . J Immunol. 2008; 181:418–430.10. Kleinjan A, Lambrecht BN. Dendritic cells in rhinitis. Handb Exp Pharmacol. 2009; 115–136.
Article11. Corthay A. A three-cell model for activation of naïve T helper cells. Scand J Immunol. 2006; 64:93–96.
Article12. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992; 176:1693–1702.
Article13. Hattori H, Okano M, Yoshino T, Akagi T, Nakayama E, Saito C, et al. Expression of costimulatory CD80/CD86-CD28/CD152 molecules in nasal mucosa of patients with perennial allergic rhinitis. Clin Exp Allergy. 2001; 31:1242–1249.
Article14. Wong CK, Lun SW, Ko FW, Ip WK, Hui DS, Lam CW. Increased expression of plasma and cell surface co-stimulatory molecules CTLA-4, CD28 and CD86 in adult patients with allergic asthma. Clin Exp Immunol. 2005; 141:122–129.
Article15. Chen ZR, Zhang GB, Wang YQ, Yan YD, Zhou WF, Zhu C, et al. Therapeutic effects of anti-B7-H3 antibody in an ovalbumin-induced mouse asthma model. Ann Allergy Asthma Immunol. 2013; 111:276–281.
Article16. Li JG, Du YM, Yan ZD, Yan J, Zhuansun YX, Chen R, et al. CD80 and CD86 knockdown in dendritic cells regulates Th1/Th2 cytokine production in asthmatic mice. Exp Ther Med. 2016; 11:878–884.
Article17. Pierrot-Deseilligny C, Souberbielle JC. Vitamin D and multiple sclerosis: an update. Mult Scler Relat Disord. 2017; 14:35–45.
Article18. Foster PS, Mould AW, Yang M, Mackenzie J, Mattes J, Hogan SP, et al. Elemental signals regulating eosinophil accumulation in the lung. Immunol Rev. 2001; 179:173–181.
Article19. KleinJan A, Willart M, van Rijt LS, Braunstahl GJ, Leman K, Jung S, et al. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006; 118:1117–1125.
Article20. Liu ZQ, Li XX, Qiu SQ, Yu Y, Li MG, Yang LT, et al. Vitamin D contributes to mast cell stabilization. Allergy. 2017; 72:1184–1192.
Article21. Yip KH, Kolesnikoff N, Yu C, Hauschild N, Taing H, Biggs L, et al. Mechanisms of vitamin D3 metabolite repression of IgE-dependent mast cell activation. J Allergy Clin Immunol. 2014; 133:1356–1364. 1364.e1–1364.e14.
Article22. El-Shazly AE, Lefebvre PP. Modulation of NK cell autocrine-induced eosinophil chemotaxis by interleukin-15 and vitamin D(3): a possible NK-eosinophil crosstalk via IL-8 in the pathophysiology of allergic rhinitis. Mediators Inflamm. 2011; 2011:373589.
Article23. Lewkowich IP, Rempel JD. HayGlass KT. Antigen-specific versus total immunoglobulin synthesis: total IgE and IgG1, but not IgG2a levels predict murine antigen-specific responses. Int Arch Allergy Immunol. 2004; 133:145–153.24. Chiu CY, Su KW, Tsai MH, Hua MC, Liao SL, Lai SH, et al. Longitudinal vitamin D deficiency is inversely related to mite sensitization in early childhood. Pediatr Allergy Immunol. 2018; 29:254–259.
Article25. Vasiliou JE, Lui S, Walker SA, Chohan V, Xystrakis E, Bush A, et al. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy. 2014; 69:1380–1389.26. Litonjua AA. Vitamin D and corticosteroids in asthma: synergy, interaction and potential therapeutic effects. Expert Rev Respir Med. 2013; 7:101–104.
Article27. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016; 37:268–272.
Article28. Chen B, Qu S, Li M, Ye L, Zhang S, Qin T, et al. Effects of 1,25-dihydroxyvitamin D3 in an ovalbumin-induced allergic rhinitis model. Int Immunopharmacol. 2017; 47:182–189.
Article29. Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA Jr, Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017; 5:881–890.
Article30. Park JH, Hong IY, Chung JW, Choi HS. Vitamin D status in South Korean population: seven-year trend from the KNHANES. Medicine (Baltimore). 2018; 97:e11032.31. Wootton AM. Improving the measurement of 25-hydroxyvitamin D. Clin Biochem Rev. 2005; 26:33–36.32. Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014; 15:323–332.
Article33. Ferreira GB, Overbergh L, Verstuyf A, Mathieu C. 1α,25-dihydroxyvitamin D3 and its analogs as modulators of human dendritic cells: a comparison dose-titration study. J Steroid Biochem Mol Biol. 2013; 136:160–165.
Article34. Ferreira GB, van Etten E, Lage K, Hansen DA, Moreau Y, Workman CT, et al. Proteome analysis demonstrates profound alterations in human dendritic cell nature by TX527, an analogue of vitamin D. Proteomics. 2009; 9:3752–3764.
Article35. Bscheider M, Butcher EC. Vitamin D immunoregulation through dendritic cells. Immunology. 2016; 148:227–236.
Article