Anat Cell Biol.
2018 Dec;51(4):274-283. 10.5115/acb.2018.51.4.274.
Quercetin induces cell death in cervical cancer by reducing O-GlcNAcylation of adenosine monophosphate-activated protein kinase
- Affiliations
-
- 1Department of Anatomy and Convergence Medical Science, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea. choiws@gnu.ac.kr
- KMID: 2430195
- DOI: http://doi.org/10.5115/acb.2018.51.4.274
Abstract
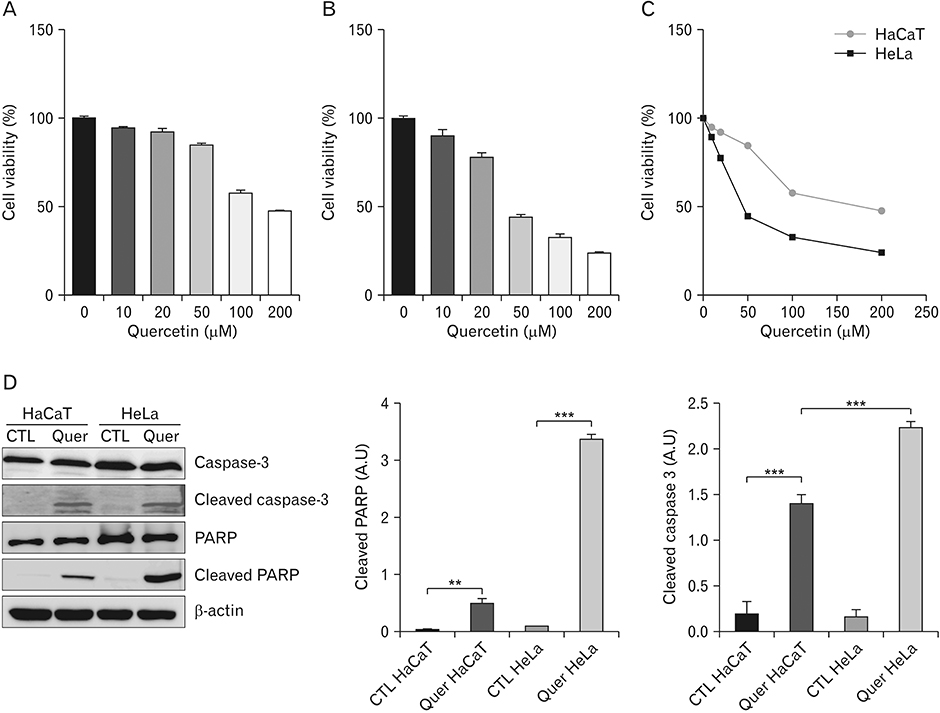
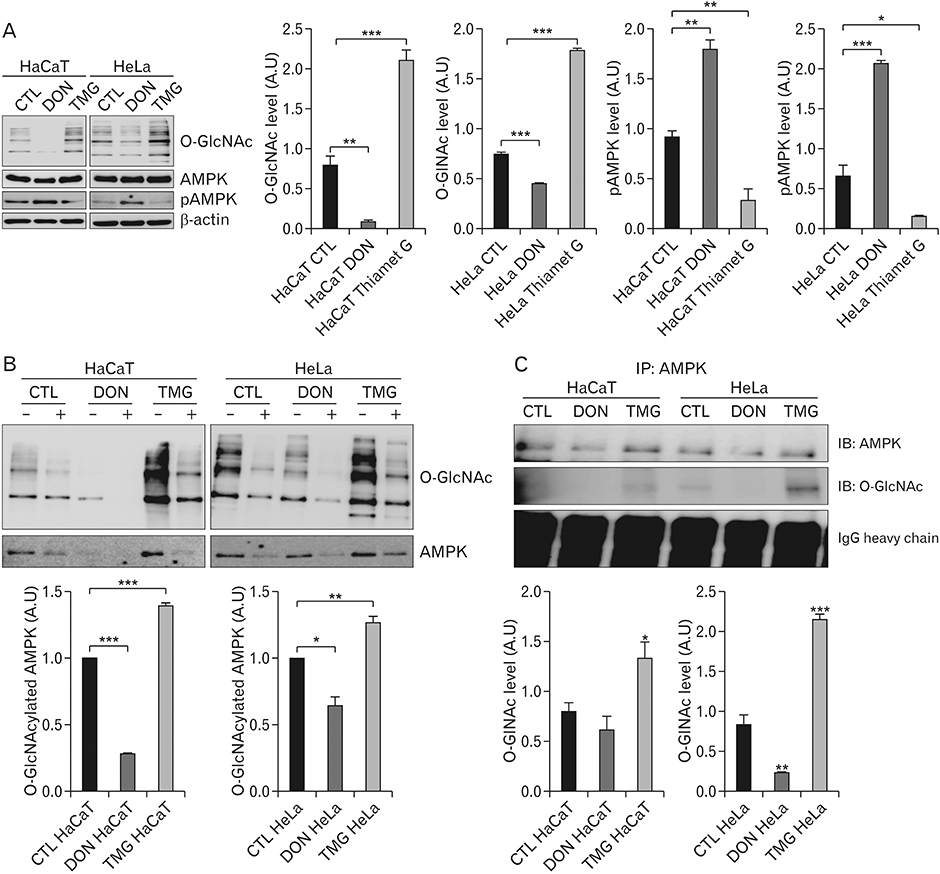
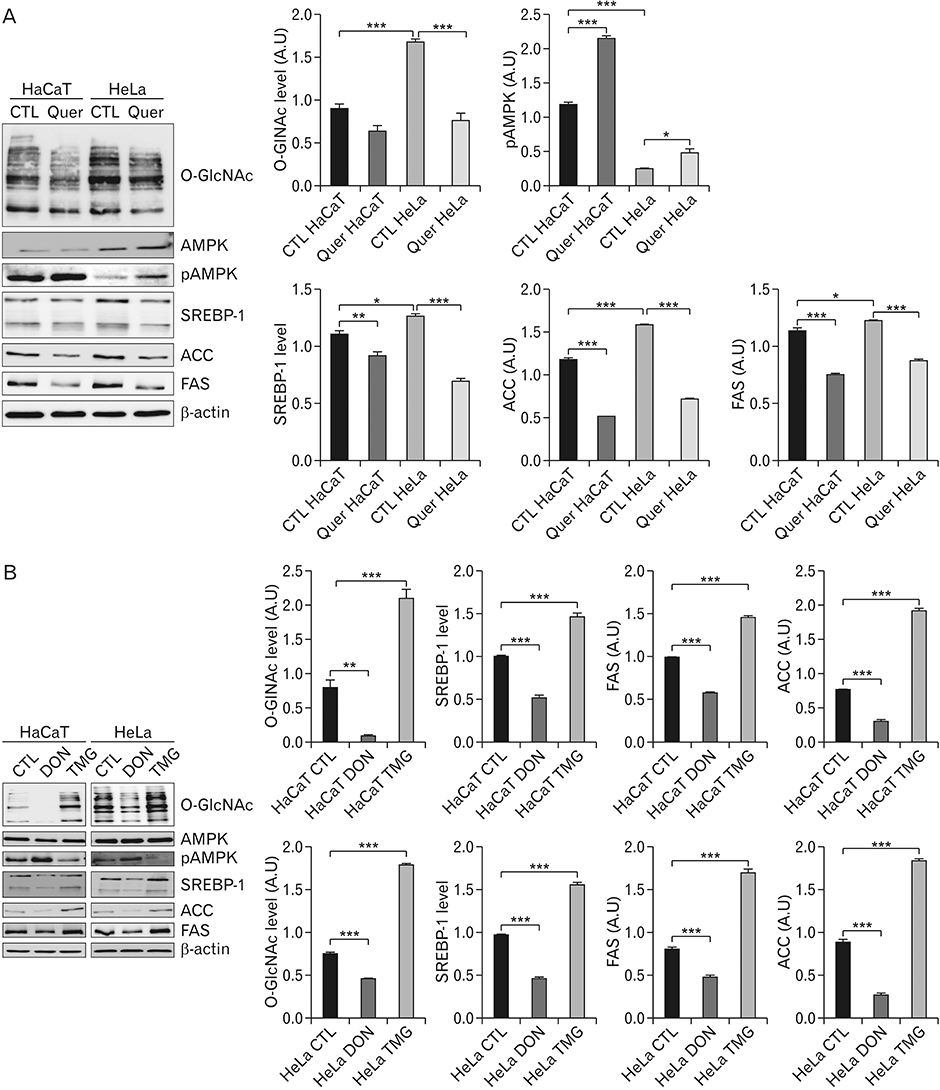
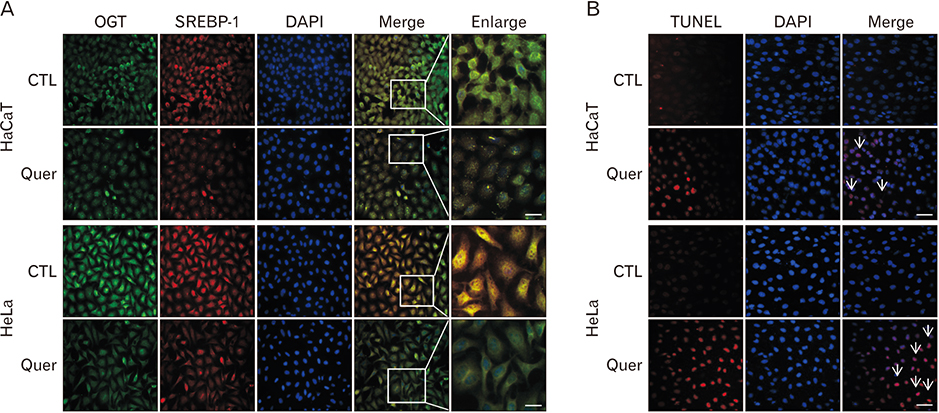
- Hyper-O-GlcNAcylation is a general feature of cancer which contributes to various cancer phenotypes, including cell proliferation and cell growth. Quercetin, a naturally occurring dietary flavonoid, has been reported to reduce the proliferation and growth of cancer. Several reports of the anticancer effect of quercetin have been published, but there is no study regarding its effect on O-GlcNAcylation. The aim of this study was to investigate the anticancer effect of quercetin on HeLa cells and compare this with its effect on HaCaT cells. Cell viability and cell death were determined by MTT and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling assays. O-GlcNAcylation of AMP-activated protein kinase (AMPK) was examined by succinylated wheat germ agglutinin pulldown and immunoprecipitation. Immunofluorescence staining was used to detect the immunoreactivitiy of O-linked N-acetylglucosamine transferase (OGT) and sterol regulatory element binding protein 1 (SREBP-1). Quercetin decreased cell proliferation and induced cell death, but its effect on HaCaT cells was lower than that on HeLa cells. O-GlcNAcylation level was higher in HeLa cells than in HaCaT cells. Quercetin decreased the expression of global O-GlcNAcylation and increased AMPK activation by reducing the O-GlcNAcylation of AMPK. AMPK activation due to reduced O-GlcNAcylation of AMPK was confirmed by treatment with 6-diazo-5-oxo-L-norleucine. Our results also demonstrated that quercetin regulated SREBP-1 and its transcriptional targets. Furthermore, immunofluorescence staining showed that quercetin treatment decreased the immunoreactivities of OGT and SREBP-1 in HeLa cells. Our findings demonstrate that quercetin exhibited its anticancer effect by decreasing the O-GlcNAcylation of AMPK. Further studies are needed to explore how quercetin regulates O-GlcNAcylation in cancer.
Keyword
MeSH Terms
-
Adenosine*
AMP-Activated Protein Kinases
Cell Death*
Cell Proliferation
Cell Survival
Deoxyuridine
Diazooxonorleucine
Fluorescent Antibody Technique
HeLa Cells
Humans
Immunoprecipitation
Phenotype
Protein Kinases*
Quercetin*
Sterol Regulatory Element Binding Protein 1
Transferases
Triticum
Uterine Cervical Neoplasms*
AMP-Activated Protein Kinases
Adenosine
Deoxyuridine
Diazooxonorleucine
Protein Kinases
Quercetin
Sterol Regulatory Element Binding Protein 1
Transferases
Figure
Cited by 1 articles
-
Cyclosporin A aggravates hydrogen peroxide-induced cell death in kidney proximal tubule epithelial cells
Daeun Moon, Jinu Kim
Anat Cell Biol. 2019;52(3):312-323. doi: 10.5115/acb.18.192.
Reference
-
1. Ma Z, Vosseller K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J Biol Chem. 2014; 289:34457–34465.
Article2. Ferrer CM, Sodi VL, Reginato MJ. O-GlcNAcylation in cancer biology: linking metabolism and signaling. J Mol Biol. 2016; 428:3282–3294.
Article3. Jiang M, Qiu Z, Zhang S, Fan X, Cai X, Xu B, Li X, Zhou J, Zhang X, Chu Y, Wang W, Liang J, Horvath T, Yang X, Wu K, Nie Y, Fan D. Elevated O-GlcNAcylation promotes gastric cancer cells proliferation by modulating cell cycle related proteins and ERK 1/2 signaling. Oncotarget. 2016; 7:61390–61402.
Article4. Moriwaki K, Asahi M. Augmented TME O-GlcNAcylation promotes tumor proliferation through the inhibition of p38 MAPK. Mol Cancer Res. 2017; 15:1287–1298.
Article5. Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is antiapoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem. 2013; 288:15121–15130.6. Ferrer CM, Lu TY, Bacigalupa ZA, Katsetos CD, Sinclair DA, Reginato MJ. O-GlcNAcylation regulates breast cancer metastasis via SIRT1 modulation of FOXM1 pathway. Oncogene. 2017; 36:559–569.
Article7. Niu Y, Xia Y, Wang J, Shi X. O-GlcNAcylation promotes migration and invasion in human ovarian cancer cells via the RhoA/ ROCK/MLC pathway. Mol Med Rep. 2017; 15:2083–2089.8. Miles SL, McFarland M, Niles RM. Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human disease. Nutr Rev. 2014; 72:720–734.
Article9. Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem Pharmacol. 2012; 83:6–15.
Article10. Li XM, Liu J, Pan FF, Shi DD, Wen ZG, Yang PL. Quercetin and aconitine synergistically induces the human cervical carcinoma HeLa cell apoptosis via endoplasmic reticulum (ER) stress pathway. PLoS One. 2018; 13:e0191062.
Article11. Kashyap D, Mittal S, Sak K, Singhal P, Tuli HS. Molecular mechanisms of action of quercetin in cancer: recent advances. Tumour Biol. 2016; 37:12927–12939.
Article12. Li X, Zhou N, Wang J, Liu Z, Wang X, Zhang Q, Liu Q, Gao L, Wang R. Quercetin suppresses breast cancer stem cells (CD44(+)/CD24(−)) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018; 196:56–62.13. Hamilton KE, Rekman JF, Gunnink LK, Busscher BM, Scott JL, Tidball AM, Stehouwer NR, Johnecheck GN, Looyenga BD, Louters LL. Quercetin inhibits glucose transport by binding to an exofacial site on GLUT1. Biochimie. 2018; 151:107–114.
Article14. Moreira L, Araújo I, Costa T, Correia-Branco A, Faria A, Martel F, Keating E. Quercetin and epigallocatechin gallate inhibit glucose uptake and metabolism by breast cancer cells by an estrogen receptor-independent mechanism. Exp Cell Res. 2013; 319:1784–1795.
Article15. Kuhajda FP. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int J Obes (Lond). 2008; 32:Suppl 4. S36–S41.
Article16. Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010; 6:457–470.
Article17. Kim I, He YY. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Front Oncol. 2013; 3:175.
Article18. Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005; 26:69–76.
Article19. Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget. 2015; 6:7365–7378.
Article20. Ishimura E, Nakagawa T, Moriwaki K, Hirano S, Matsumori Y, Asahi M. Augmented O-GlcNAcylation of AMP-activated kinase promotes the proliferation of LoVo cells, a colon cancer cell line. Cancer Sci. 2017; 108:2373–2382.21. Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN, Reginato MJ. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014; 54:820–831.
Article22. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in dietinduced insulin-resistant mice. Cell Metab. 2011; 13:376–388.
Article23. Wen YA, Xiong X, Zaytseva YY, Napier DL, Vallee E, Li AT, Wang C, Weiss HL, Evers BM, Gao T. Downregulation of SREBP inhibits tumor growth and initiation by altering cellular metabolism in colon cancer. Cell Death Dis. 2018; 9:265.
Article24. Li X, Chen YT, Hu P, Huang WC. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol Cancer Ther. 2014; 13:855–866.
Article25. Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM, Leung TH, Wong OG, Cheung AN, Ngan HY. AMPK activators suppress cervical cancer cell growth through inhibition of DVL3 mediated Wnt/beta-catenin signaling activity. PLoS One. 2013; 8:e53597.26. Yung MM, Chan DW, Liu VW, Yao KM, Ngan HY. Activation of AMPK inhibits cervical cancer cell growth through AKT/ FOXO3a/FOXM1 signaling cascade. BMC Cancer. 2013; 13:327.
Article27. Chahar MK, Sharma N, Dobhal MP, Joshi YC. Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev. 2011; 5:1–12.28. Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech. 2013; 3:439–459.
Article29. Liu Y, Ren Y, Cao Y, Huang H, Wu Q, Li W, Wu S, Zhang J. Discovery of a low toxicity O-GlcNAc transferase (OGT) inhibitor by structure-based virtual screening of natural products. Sci Rep. 2017; 7:12334.
Article30. Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011; 80:825–858.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
- Effect of Cyclic Adenosine Monophosphate and Insulin on the Hormone Secretion of In Vitro Normal Placental Tissue according to the Number of Gestational Weeks
- Adenosine monophosphate-activated protein kinase in diabetic nephropathy
- AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin
- Quercetin-induced apoptosis ameliorates vascular smooth muscle cell senescence through AMP-activated protein kinase signaling pathway