Yonsei Med J.
2018 Nov;59(9):1096-1106. 10.3349/ymj.2018.59.9.1096.
Inhibition of miR-128 Abates Aβ-Mediated Cytotoxicity by Targeting PPAR-γ via NF-κB Inactivation in Primary Mouse Cortical Neurons and Neuro2a Cells
- Affiliations
-
- 1Department of Rehabilitation Medicine, Huaihe Hospital of Henan University, Kaifeng, China. angelcindtg@yahoo.com
- 2Department of Neurology, Huaihe Hospital of Henan University, Kaifeng, China.
- KMID: 2422495
- DOI: http://doi.org/10.3349/ymj.2018.59.9.1096
Abstract
- PURPOSE
Alzheimer's disease (AD) is the sixth most common cause of death in the United States. MicroRNAs have been identified as vital players in neurodegenerative diseases, including AD. microRNA-128 (miR-128) has been shown to be dysregulated in AD. This study aimed to explore the roles and molecular mechanisms of miR-128 in AD progression.
MATERIALS AND METHODS
Expression patterns of miR-128 and peroxisome proliferator-activated receptor gamma (PPAR-γ) messenger RNA in clinical samples and cells were measured using RT-qPCR assay. PPAR-γ protein levels were determined by Western blot assay. Cell viability was determined by MTT assay. Cell apoptotic rate was detected by flow cytometry via double-staining of Annexin V-FITC/PI. Caspase 3 and NF-κB activity was determined by a Caspase 3 Activity Assay Kit or NF-κB p65 Transcription Factor Assay Kit, respectively. Bioinformatics prediction and luciferase reporter assay were used to investigate interactions between miR-128 and PPAR-γ 3"²UTR.
RESULTS
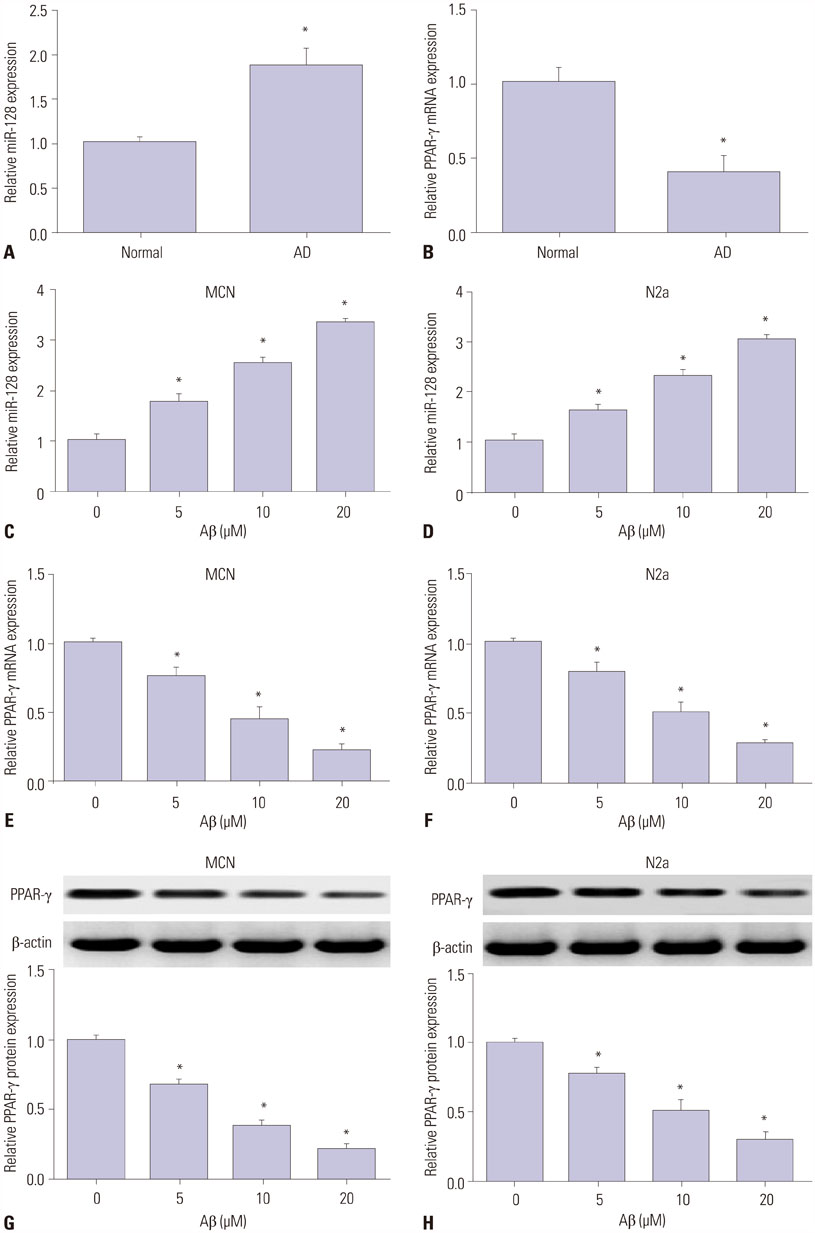
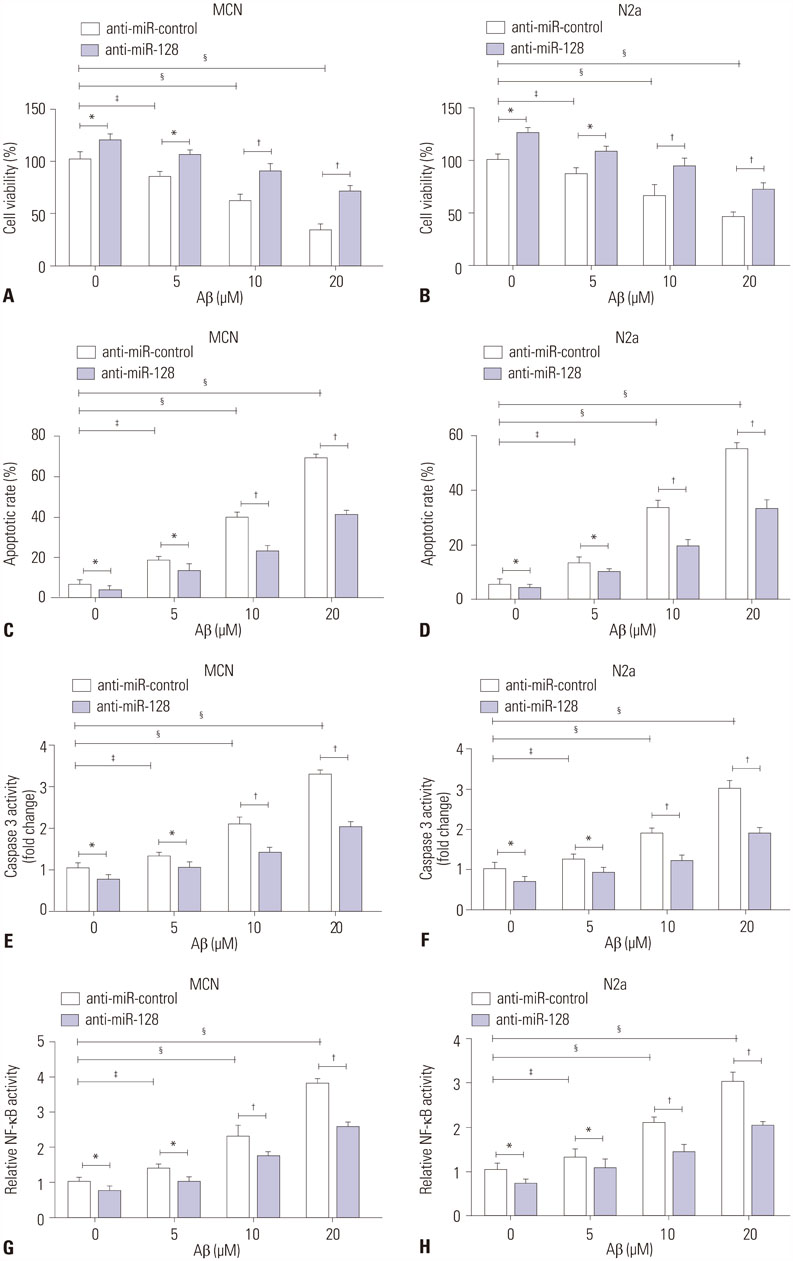
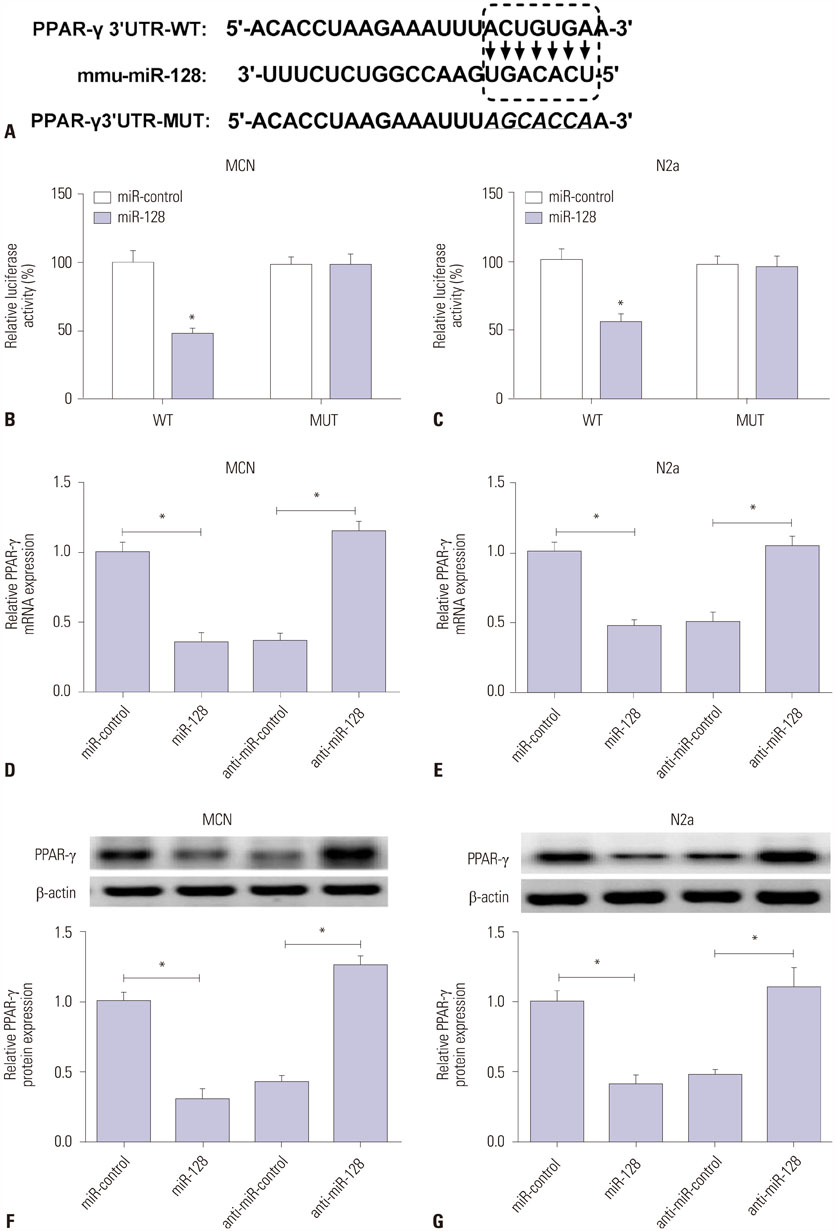
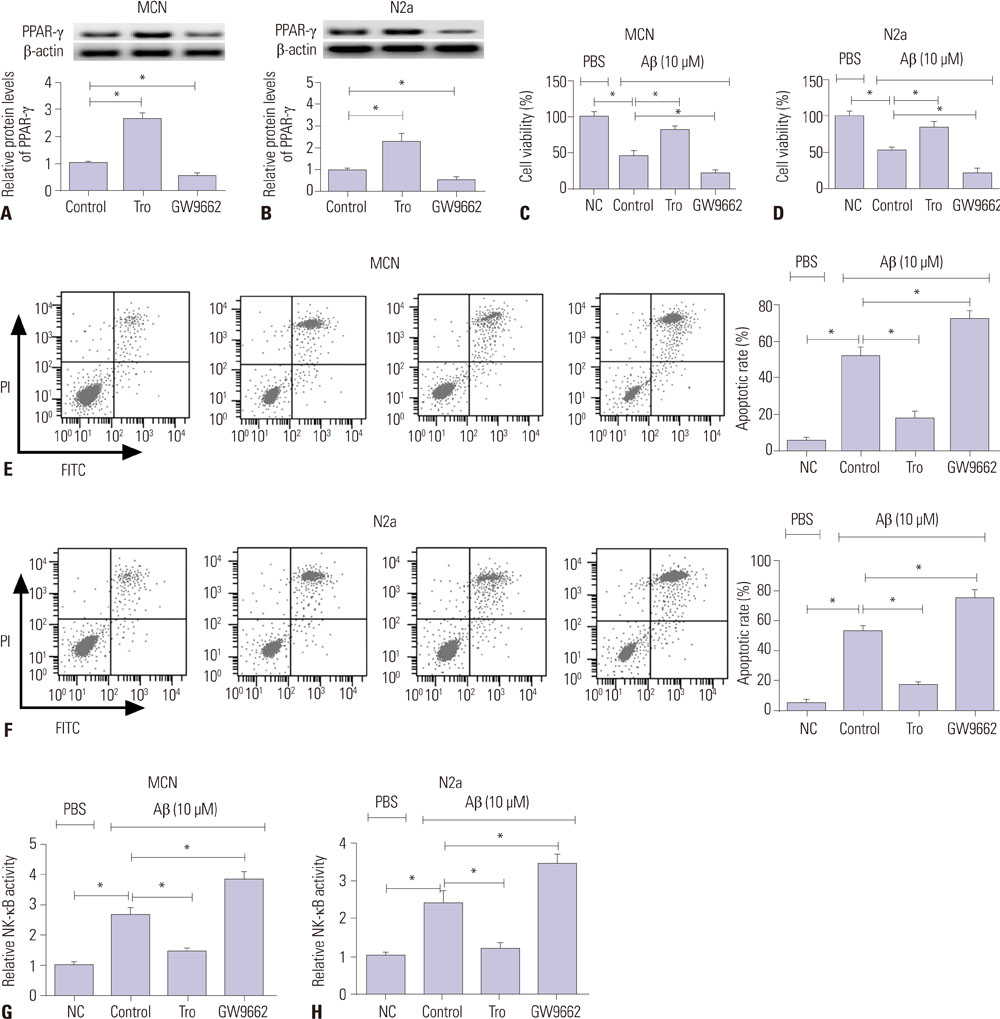
MiR-128 expression was upregulated and PPAR-γ expression was downregulated in plasma from AD patients and amyloid-β (Aβ)-treated primary mouse cortical neurons (MCN) and Neuro2a (N2a) cells. Inhibition of miR-128 decreased Aβ-mediated cytotoxicity through inactivation of NF-κB in MCN and N2a cells. Moreover, PPAR-γ was a target of miR-128. PPAR-γ upregulation attenuated Aβ-mediated cytotoxicity by inactivating NF-κB in MCN and N2a cells. Furthermore, PPAR-γ downregulation was able to abolish the effect of anti-miR-128 on cytotoxicity and NF-κB activity in MCN and N2a cells.
CONCLUSION
MiR-128 inhibitor decreased Aβ-mediated cytotoxicity by upregulating PPAR-γ via inactivation of NF-κB in MCN and N2a cells, providing a new potential target in AD treatment.
Keyword
MeSH Terms
-
Alzheimer Disease
Animals
Blotting, Western
Caspase 3
Cause of Death
Cell Survival
Computational Biology
Down-Regulation
Flow Cytometry
Humans
Luciferases
Mice*
MicroRNAs
Neurodegenerative Diseases
Neurons*
Plasma
PPAR gamma
RNA, Messenger
Transcription Factor RelA
United States
Up-Regulation
Caspase 3
Luciferases
MicroRNAs
PPAR gamma
RNA, Messenger
Transcription Factor RelA
Figure
Reference
-
1. Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016; 12:459–509.2. Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014; 88:640–651.
Article3. Kumar A, Singh A, Ekavali . A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacol Rep. 2015; 67:195–203.
Article4. Kim DH, Yeo SH, Park JM, Choi JY, Lee TH, Park SY, et al. Genetic markers for diagnosis and pathogenesis of Alzheimer's disease. Gene. 2014; 545:185–193.
Article5. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016; 8:595–608.
Article6. Kang S, Lee YH, Lee JE. Metabolism-Centric Overview of the Pathogenesis of Alzheimer's Disease. Yonsei Med J. 2017; 58:479–488.
Article7. Mémet S. NF-kappaB functions in the nervous system: from development to disease. Biochem Pharmacol. 2006; 72:1180–1195.8. Shi ZM, Han YW, Han XH, Zhang K, Chang YN, Hu ZM, et al. Upstream regulators and downstream effectors of NF-κB in Alzheimer's disease. J Neurol Sci. 2016; 366:127–134.
Article9. Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009; 7:147–154.
Article10. Karnati HK, Panigrahi MK, Gutti RK, Greig NH, Tamargo IA. miRNAs: key players in neurodegenerative disorders and epilepsy. J Alzheimers Dis. 2015; 48:563–580.
Article11. Adlakha YK, Saini N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. Mol Cancer. 2014; 13:33.
Article12. Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport. 2007; 18:297–300.
Article13. Müller M, Kuiperij HB, Claassen JA, Küsters B, Verbeek MM. MicroRNAs in Alzheimer's disease: differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol Aging. 2014; 35:152–158.
Article14. Jiang Q, Heneka M, Landreth GE. The role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in Alzheimer's disease: therapeutic implications. CNS Drugs. 2008; 22:1–14.
Article15. Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, et al. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci U S A. 2006; 103:443–448.
Article16. Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer's disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000; 20:558–567.
Article17. Fakhfouri G, Ahmadiani A, Rahimian R, Grolla AA, Moradi F, Haeri A. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway. Neuropharmacology. 2012; 63:653–666.
Article18. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014; 13:614–629.
Article19. Schmidt K. Clinical dementia rating scale. In : Michalos AC, editor. Encyclopedia of quality of life and well-being research. Dordrecht: Springer;2014. p. 957–960.20. Mitchell AJ. The Mini-Mental State Examination (MMSE): update on its diagnostic accuracy and clinical utility for cognitive disorders. In : Larner AJ, editor. Cognitive screening instruments. Cham: Springer;2017. p. 37–48.21. Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001; 32:683–696.22. Chen YC, Wu JS, Tsai HD, Huang CY, Chen JJ, Sun GY, et al. Peroxisome proliferator-activated receptor gamma (PPAR-γ) and neurodegenerative disorders. Mol Neurobiol. 2012; 46:114–124.
Article23. Reddy PH, Tonk S, Kumar S, Vijayan M, Kandimalla R, Kuruva CS, et al. A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer’s disease. Biochem Biophys Res Commun. 2017; 483:1156–1165.
Article24. Femminella GD, Ferrara N, Rengo G. The emerging role of microRNAs in Alzheimer's disease. Front Physiol. 2015; 6:40.
Article25. Millan MJ. Linking deregulation of non-coding RNA to the core pathophysiology of Alzheimer's disease: an integrative review. Prog Neurobiol. 2017; 156:1–68.
Article26. Long JM, Ray B, Lahiri DK. MicroRNA-153 physiologically inhibits expression of amyloid-β precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J Biol Chem. 2012; 287:31298–31310.
Article27. Absalon S, Kochanek DM, Raghavan V, Krichevsky AM. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tauphosphorylation, and apoptosis in postmitotic neurons. J Neurosci. 2013; 33:14645–14659.
Article28. McSweeney KM, Gussow AB, Bradrick SS, Dugger SA, Gelfman S, Wang Q, et al. Inhibition of microRNA 128 promotes excitability of cultured cortical neuronal networks. Genome Res. 2016; 26:1411–1416.
Article29. Tiribuzi R, Crispoltoni L, Porcellati S, Di Lullo M, Florenzano F, Pirro M, et al. miR128 up-regulation correlates with impaired amyloid β(1-42) degradation in monocytes from patients with sporadic Alzheimer's disease. Neurobiol Aging. 2014; 35:345–356.
Article30. Guidi M, Muiños-Gimeno M, Kagerbauer B, Martí E, Estivill X, Espinosa-Parrilla Y. Overexpression of miR-128 specifically inhibits the truncated isoform of NTRK3 and upregulates BCL2 in SHSY5Y neuroblastoma cells. BMC Mol Biol. 2010; 11:95.
Article31. Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1997; 94:2642–2647.
Article32. Valerio A, Boroni F, Benarese M, Sarnico I, Ghisi V, Bresciani LG, et al. NF-kappaB pathway: a target for preventing beta-amyloid (Abeta)-induced neuronal damage and Abeta42 production. Eur J Neurosci. 2006; 23:1711–1720.
Article33. Lin W, Ding M, Xue J, Leng W. The role of TLR2/JNK/NF-κB pathway in amyloid β peptide-induced inflammatory response in mouse NG108-15 neural cells. Int Immunopharmacol. 2013; 17:880–884.
Article34. Villapol S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell Mol Neurobiol. 2018; 38:121–132.
Article35. Corona JC, Duchen MR. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic Biol Med. 2016; 100:153–163.
Article36. Lezana JP, Dagan SY, Robinson A, Goldstein RS, Fainzilber M, Bronfman FC, et al. Axonal PPARγ promotes neuronal regeneration after injury. Dev Neurobiol. 2016; 76:688–701.
Article37. Landreth G, Jiang Q, Mandrekar S, Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer's disease. Neurotherapeutics. 2008; 5:481–489.
Article38. Toba J, Nikkuni M, Ishizeki M, Yoshii A, Watamura N, Inoue T, et al. PPARγ agonist pioglitazone improves cerebellar dysfunction at pre-Aβ deposition stage in APPswe/PS1dE9 Alzheimer's disease model mice. Biochem Biophys Res Commun. 2016; 473:1039–1044.
Article39. de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. J Alzheimers Dis. 2006; 9:167–181.
Article40. Kitamura Y, Shimohama S, Koike H, Kakimura Ji, Matsuoka Y, Nomura Y, et al. Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer's disease brains. Biochem Biophys Res Commun. 1999; 254:582–586.
Article41. Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M. Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Exp Cell Res. 2005; 304:91–104.
Article42. Sastre M, Dewachter I, Landreth GE, Willson TM, Klockgether T, van Leuven F, et al. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J Neurosci. 2003; 23:9796–9804.
Article43. Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B. Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J Neurosci. 2004; 24:10908–10917.
Article44. d'Abramo C, Massone S, Zingg JM, Pizzuti A, Marambaud P, Dalla Piccola B, et al. Role of peroxisome proliferator-activated receptor gamma in amyloid precursor protein processing and amyloid beta-mediated cell death. Biochem J. 2005; 391(Pt 3):693–698.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- miR-215 Enhances HCV Replication by Targeting TRIM22 and Inactivating NF-κB Signaling
- Galangin Suppresses Pro-Inflammatory Gene Expression in Polyinosinic-Polycytidylic Acid-Stimulated Microglial Cells
- MiR-506 Promotes Natural Killer Cell Cytotoxicity against Human Hepatocellular Carcinoma Cells by Targeting STAT3
- Long Noncoding RNA NEAT1 Aggravates Aβ-Induced Neuronal Damage by Targeting miR-107 in Alzheimer's Disease
- MiR-590 Inhibits Endothelial Cell Apoptosis by Inactivating the TLR4/NF-κB Pathway in Atherosclerosis