Nutr Res Pract.
2018 Aug;12(4):275-282. 10.4162/nrp.2018.12.4.275.
Effect of isoflavone-enriched whole soy milk powder supplementation on bone metabolism in ovariectomized mice
- Affiliations
-
- 1Center for Efficacy Assessment and Development of Functional Foods and Drugs, Hallym University, 1 Hallymdaehak-gil, Chuncheon, Gangwon 24252, Korea. myej4@hallym.ac.kr
- 2Department of Food Science & Nutrition, Dongseo University, Busan 47011, Korea.
- 3Institute of Food Processing Technology, Uwell Bio Co. Ltd., Gangwon 25451, Korea.
- 4Department of Biochemistry, College of Medicine, Hallym University, Gangwon 24252, Korea.
- KMID: 2420992
- DOI: http://doi.org/10.4162/nrp.2018.12.4.275
Abstract
- BACKGROUND
/OBJECTIVE: There is intense interest in soy isoflavone as a hormone replacement therapy for the prevention of postmenopausal osteoporosis. A new kind of isoflavone-enriched whole soy milk powder (I-WSM) containing more isoflavones than conventional whole soy milk powder was recently developed. The aim of this study was to investigate the effects of I-WSM on bone metabolism in ovariectomized mice.
MATERIALS/METHODS
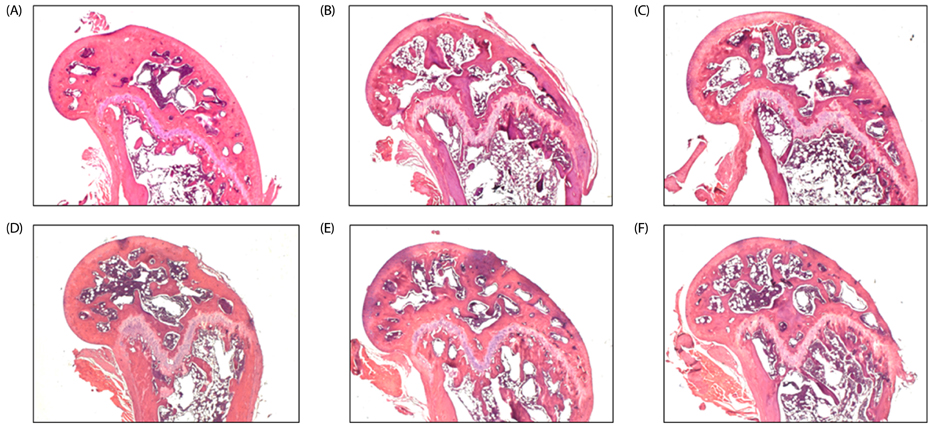
Sixty female ICR mice individually underwent ovariectomy (OVX) or a sham operation, and were randomized into six groups of 10 animals each as follows: Sham, OVX, OVX with 2% I-WSM diet, OVX with 10% I-WSM diet, OVX with 20% I-WSM diet, and OVX with 20% WSM diet. After an 8-week treatment period, bone mineral density (BMD), calcium, alkaline phosphatase (ALP), tartrate-resistant acid phosphatase (TRAP) 5b, osteocalcin (OC), procollagen 1 N-terminal propeptide (P1NP), and osteoprotegenin (OPG) were analyzed.
RESULTS
BMD was significantly lower in the OVX group compared to the Sham group but was significantly higher in OVX + 10% I-WSM and OVX + 20% I-WSM groups compared to the OVX group (P < 0.05). Serum calcium concentration significantly increased in the OVX + 10% and 20% I-WSM groups. Serum ALP levels were significantly lower in the OVX + 10% and 20% I-WSM groups compared to the other experimental groups (P < 0.05). OC was significantly reduced in the OVX group compared to the Sham group (P < 0.05), but a dose-dependent increase was observed in the OVX groups supplemented with I-WSM. P1NP and OPG levels were significantly reduced, while TRAP 5b level was significantly elevated in the OVX group compared with the Sham group, which was not affected by I-WSM (P < 0.05).
CONCLUSIONS
This study suggests that I-WSM supplementation in OVX mice has the effect of preventing BMD reduction and promoting bone formation. Therefore, I-WSM can be used as an effective alternative to postmenopausal osteoporosis prevention.
Keyword
MeSH Terms
-
Acid Phosphatase
Alkaline Phosphatase
Animals
Bone Density
Bone Remodeling
Calcium
Diet
Female
Functional Food
Hormone Replacement Therapy
Humans
Isoflavones
Metabolism*
Mice*
Mice, Inbred ICR
Osteocalcin
Osteogenesis
Osteoporosis, Postmenopausal
Ovariectomy
Procollagen
Soy Milk*
Soybeans
Acid Phosphatase
Alkaline Phosphatase
Calcium
Isoflavones
Osteocalcin
Procollagen
Figure
Reference
-
1. Lecart MP, Reginster JY. Current options for the management of postmenopausal osteoporosis. Expert Opin Pharmacother. 2011; 12:2533–2552.
Article2. Seckin B, Pekcan MK, Inal HA, Gulerman C. The relationship between breast density and bone mineral density in never users of postmenopausal hormone therapy. Aging Clin Exp Res. 2017; 29:537–541.
Article3. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002; 288:321–333.
Article4. Gambacciani M, Levancini M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Prz Menopauzalny. 2014; 13:213–220.5. Qi S, Zheng H. Combined effects of phytoestrogen genistein and silicon on ovariectomy-induced bone loss in rat. Biol Trace Elem Res. 2017; 177:281–287.
Article6. Pilšáková L, Riečanský I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010; 59:651–664.
Article7. Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013; 38:15–25.
Article8. Moutsatsou P. The spectrum of phytoestrogens in nature: our knowledge is expanding. Hormones (Athens). 2007; 6:173–193.9. Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck AJ. Phytoestrogens: recent developments. Planta Med. 2003; 69:589–599.
Article10. Huang HY, Yang HP, Yang HT, Yang TC, Shieh MJ, Huang SY. One-year soy isoflavone supplementation prevents early postmenopausal bone loss but without a dose-dependent effect. J Nutr Biochem. 2006; 17:509–517.
Article11. Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001; 86:5217–5221.
Article12. Zhang X, Shu XO, Li H, Yang G, Li Q, Gao YT, Zheng W. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005; 165:1890–1895.
Article13. Kim MS, Lee YS. Effects of soy isoflavone and/or estrogen treatments on bone metabolism in ovariectomized rats. J Med Food. 2005; 8:439–445.
Article14. Bitto A, Burnett BP, Polito F, Marini H, Levy RM, Armbruster MA, Minutoli L, Di Stefano V, Irrera N, Antoci S, Granese R, Squadrito F, Altavilla D. Effects of genistein aglycone in osteoporotic, ovariectomized rats: a comparison with alendronate, raloxifene and estradiol. Br J Pharmacol. 2008; 155:896–905.
Article15. Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006; 295:2057–2071.16. Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005; 115:3318–3325.
Article17. Bergh JJ, Xu Y, Farach-Carson MC. Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel. Endocrinology. 2004; 145:426–436.
Article18. Klepzig M, Jonas D, Oremek GM. Procollagen type 1 amino-terminal propeptide: a marker for bone metastases in prostate carcinoma. Anticancer Res. 2009; 29:671–673.19. Ljusberg J, Wang Y, Lång P, Norgård M, Dodds R, Hultenby K, Ek-Rylander B, Andersson G. Proteolytic excision of a repressive loop domain in tartrate-resistant acid phosphatase by cathepsin K in osteoclasts. J Biol Chem. 2005; 280:28370–28381.
Article20. Campbell SE, Febbraio MA. Effects of ovarian hormones on exercise metabolism. Curr Opin Clin Nutr Metab Care. 2001; 4:515–520.
Article21. Misso ML, Jang C, Adams J, Tran J, Murata Y, Bell R, Boon WC, Simpson ER, Davis SR. Differential expression of factors involved in fat metabolism with age and the menopause transition. Maturitas. 2005; 51:299–306.
Article22. Huang S, Chen JC, Hsu CW, Chang WH. Effects of nano calcium carbonate and nano calcium citrate on toxicity in ICR mice and on bone mineral density in an ovariectomized mice model. Nanotechnology. 2009; 20:375102.
Article23. Zoth N, Weigt C, Laudenbach-Leschowski U, Diel P. Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. J Steroid Biochem Mol Biol. 2010; 122:100–105.
Article24. Zheng W, Rogoschin J, Niehoff A, Oden K, Kulling SE, Xie M, Diel P. Combinatory effects of phytoestrogens and exercise on body fat mass and lipid metabolism in ovariectomized female rats. J Steroid Biochem Mol Biol. 2018; 178:73–81.
Article25. Wu J, Wang X, Chiba H, Higuchi M, Nakatani T, Ezaki O, Cui H, Yamada K, Ishimi Y. Combined intervention of soy isoflavone and moderate exercise prevents body fat elevation and bone loss in ovariectomized mice. Metabolism. 2004; 53:942–948.
Article26. Hertrampf T, Gruca MJ, Seibel J, Laudenbach U, Fritzemeier KH, Diel P. The bone-protective effect of the phytoestrogen genistein is mediated via ER α-dependent mechanisms and strongly enhanced by physical activity. Bone. 2007; 40:1529–1535.
Article27. Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med. 2011; 171:1363–1369.
Article28. Wang H, Murphy PA. Isoflavone content in commercial soybean foods. J Agric Food Chem. 1994; 42:1666–1673.
Article29. Davey RA, Hahn CN, May BK, Morris HA. Osteoblast gene expression in rat long bones: effects of ovariectomy and dihydrotestosterone on mRNA levels. Calcif Tissue Int. 2000; 67:75–79.
Article30. Nishizawa Y, Nakamura T, Ohta H, Kushida K, Gorai I, Shiraki M, Fukunaga M, Hosoi T, Miki T, Chaki O, Ichimura S, Nakatsuka K, Miura M. Committee on the Guidelines for the Use of Biochemical Markers of Bone Turnover in Osteoporosis Japan Osteoporosis Society. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J Bone Miner Metab. 2005; 23:97–104.
Article31. Taku K, Melby MK, Kurzer MS, Mizuno S, Watanabe S, Ishimi Y. Effects of soy isoflavone supplements on bone turnover markers in menopausal women: systematic review and meta-analysis of randomized controlled trials. Bone. 2010; 47:413–423.
Article32. Fu SW, Zeng GF, Zong SH, Zhang ZY, Zou B, Fang Y, Lu L, Xiao DQ. Systematic review and meta-analysis of the bone protective effect of phytoestrogens on osteoporosis in ovariectomized rats. Nutr Res. 2014; 34:467–477.
Article33. Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005; 26:97–122.34. Vasikaran SD. Utility of biochemical markers of bone turnover and bone mineral density in management of osteoporosis. Crit Rev Clin Lab Sci. 2008; 45:221–258.
Article35. Miller PD, Hochberg MC, Wehren LE, Ross PD, Wasnich RD. How useful are measures of BMD and bone turnover? Curr Med Res Opin. 2005; 21:545–553.
Article36. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001; 142:5050–5055.
Article37. Bateman TA, Countryman S. Osteoprotegerin and bone loss associated with spaceflight. Drug Discov Today. 2002; 7:456–457.38. Brink E, Coxam V, Robins S, Wahala K, Cassidy A, Branca F. PHYTOS Investigator. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr. 2008; 87:761–770.
Article39. Lydeking-Olsen E, Beck-Jensen JE, Setchell KD, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss-a 2 year randomized, placebo-controlled trial. Eur J Nutr. 2004; 43:246–257.40. Nikander E, Metsä-Heikkilä M, Ylikorkala O, Tiitinen A. Effects of phytoestrogens on bone turnover in postmenopausal women with a history of breast cancer. J Clin Endocrinol Metab. 2004; 89:1207–1212.
Article41. Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Väänänen HK. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000; 15:1337–1345.
Article42. Halleen JM. Tartrate-resistant acid phosphatase 5B is a specific and sensitive marker of bone resorption. Anticancer Res. 2003; 23:1027–1029.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Genistein and Soy Protein on Lipids Metabolism in Ovariectomized Rats
- The Effect of Isoflavone and/or Grape Seed Oil Supplementation on Blood Lipid Profiles and Bone Strength in Ovariectomized Female Rats
- Combined effects of soy isoflavone and lecithin on bone loss in ovariectomized mice
- Calcium and Phosphorus Balance Study by Soy Isoflavone Intake in Ovariectomized Rats
- Relation between Health Examination Outcome and Intake of Soy Food and Isoflavone among adult male in Seoul