Korean Circ J.
2018 Oct;48(10):933-943. 10.4070/kcj.2017.0373.
Treatment with 3-Bromo-4,5-Dihydroxybenzaldehyde Improves Cardiac Function by Inhibiting Macrophage Infiltration in Mice
- Affiliations
-
- 1Department of Cardiology, Yiwu Hospital of Wenzhou Medical University (Yiwu Central Hospital), Yiwu, China. 1793870950@qq.com
- KMID: 2420646
- DOI: http://doi.org/10.4070/kcj.2017.0373
Abstract
- BACKGROUND AND OBJECTIVES
Appropriate inflammatory response is necessary for cardiac repairing after acute myocardial infarction (MI). Three-Bromo-4,5-dihydroxybenzaldehyde (BDB) is a potent antioxidant and natural bromophenol compound derived from red algae. Although BDB has been shown to have an anti-inflammatory effect, it remains unclear whether BDB affects cardiac remolding after MI. The aim of this study was to investigate the potential role of BDB on cardiac function recovery after MI in mice.
METHODS
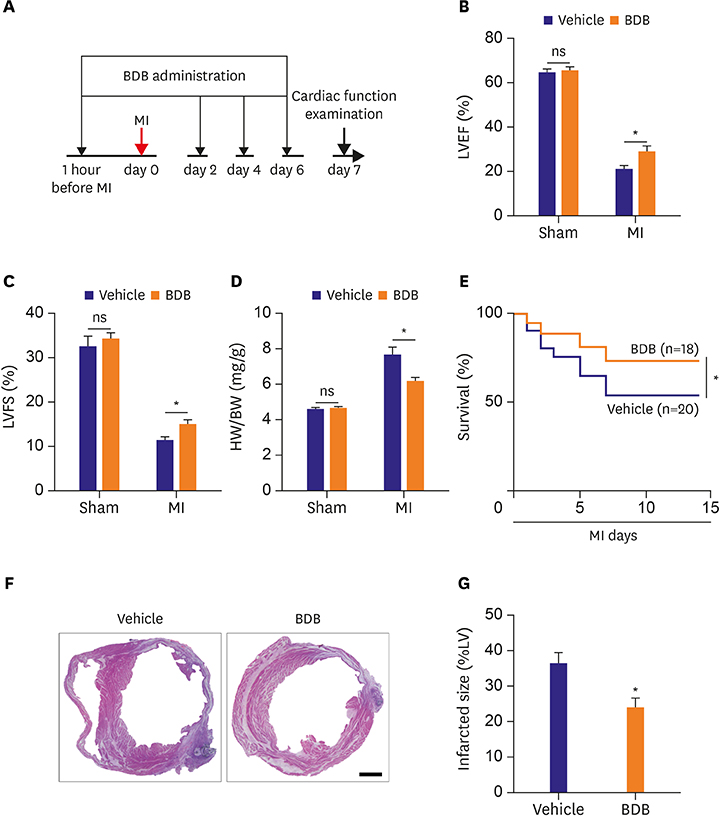
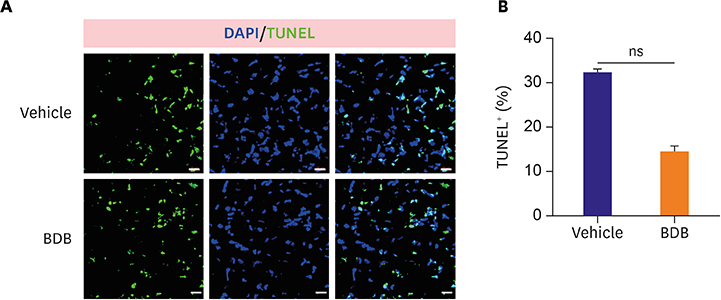
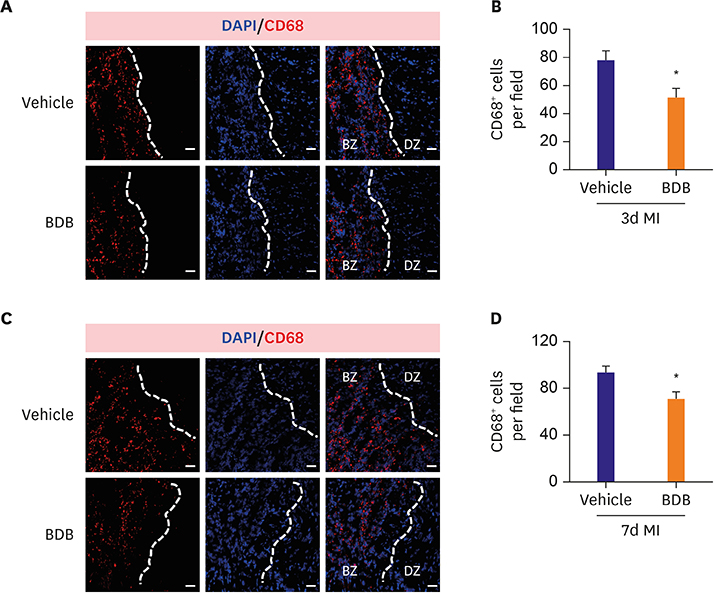
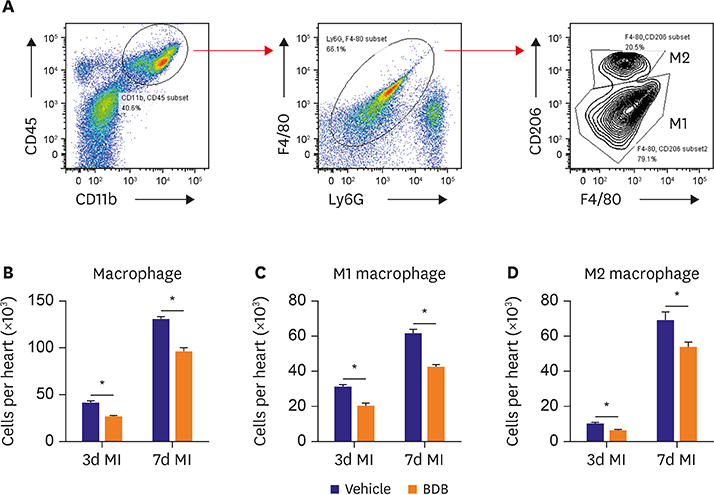
Mice were intraperitoneally injected with BDB (100 mg/kg) or vehicle control respectively 1 hour before MI and then treated every other day. Cardiac function was monitored by transthoracic echocardiography at day 7 after MI. The survival of mice was observed for 2 weeks and hematoxylin and eosin (H&E) staining was used to determine the infarct size. Macrophages infiltration was examined by immunofluorescence staining. Enzyme-linked immunosorbent assay (ELISA) was used to test the production of cytokines associated with macrophages. The phosphorylation status of nuclear factor (NF)-κB was determined by western blot.
RESULTS
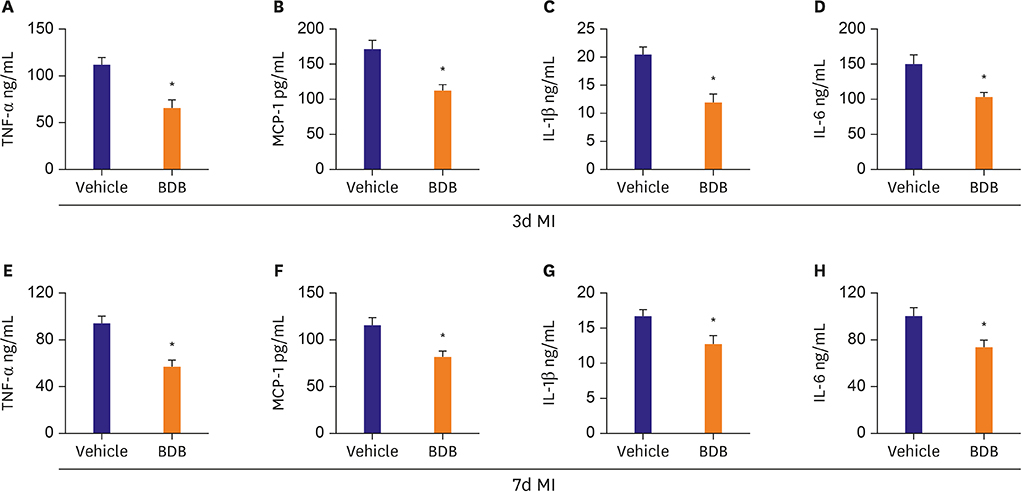
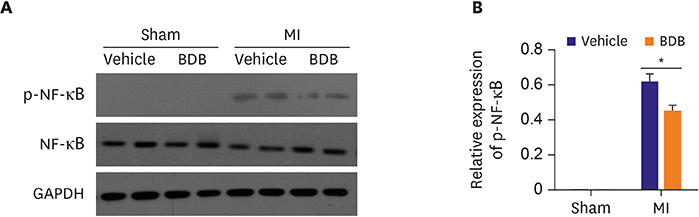
BDB administration dramatically improved cardiac function recovery, and decreased mortality and infarcted size after MI. Treatment with BDB reduced CD68+ macrophages, M1 and M2 macrophages infiltration post-MI, and suppressed the secretion of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, monocyte chemoattractant protein (MCP)-1, and IL-6 in the injured hearts. Furthermore, BDB inhibited the phosphorylation of NF-κB in the infarcted hearts.
CONCLUSIONS
These data demonstrate, for the first time, that BDB treatment facilitated cardiac healing by suppressing pro-inflammatory cytokine secretion, and indicate that BDB may serve as a therapeutic agent for acute MI.
MeSH Terms
-
Animals
Blotting, Western
Cytokines
Echocardiography
Enzyme-Linked Immunosorbent Assay
Eosine Yellowish-(YS)
Fluorescent Antibody Technique
Heart
Hematoxylin
Interleukin-6
Interleukins
Macrophages*
Mice*
Monocytes
Mortality
Myocardial Infarction
Phosphorylation
Recovery of Function
Rhodophyta
Tumor Necrosis Factor-alpha
Cytokines
Eosine Yellowish-(YS)
Hematoxylin
Interleukin-6
Interleukins
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Aurora AB, Porrello ER, Tan W, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014; 124:1382–1392.
Article2. Maruotti N, Annese T, Cantatore FP, Ribatti D. Macrophages and angiogenesis in rheumatic diseases. Vasc Cell. 2013; 5:11.
Article3. Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res. 2015; 116:354–367.
Article4. Fan X, Xu NJ, Shi JG. Bromophenols from the red alga Rhodomela confervoides . J Nat Prod. 2003; 66:455–458.5. Li K, Li XM, Ji NY, Wang BG. Bromophenols from the marine red alga Polysiphonia urceolata with DPPH radical scavenging activity. J Nat Prod. 2008; 71:28–30.6. Kim SY, Kim SR, Oh MJ, Jung SJ, Kang SY. In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J Microbiol. 2011; 49:102–106.7. Piao MJ, Kang KA, Ryu YS, et al. The red algae compound 3-bromo-4,5-dihydroxybenzaldehyde protects human keratinocytes on oxidative stress-related molecules and pathways activated by UVB irradiation. Mar Drugs. 2017; 15:E268.
Article8. Hyun YJ, Piao MJ, Zhang R, Choi YH, Chae S, Hyun JW. Photo-protection by 3-bromo-4, 5-dihydroxybenzaldehyde against ultraviolet B-induced oxidative stress in human keratinocytes. Ecotoxicol Environ Saf. 2012; 83:71–78.
Article9. Kim KC, Hyun YJ, Hewage SR, et al. 3-Bromo-4,5-dihydroxybenzaldehyde enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar Drugs. 2017; 15:E291.
Article10. Kang NJ, Han SC, Kang HJ, et al. Anti-inflammatory effect of 3-bromo-4,5-dihydroxybenzaldehyde, a component of polysiphonia morrowii, in vivo and in vitro. Toxicol Res. 2017; 33:325–332.11. Kong D, Shen Y, Liu G, et al. PKA regulatory IIα subunit is essential for PGD2-mediated resolution of inflammation. J Exp Med. 2016; 213:2209–2226.
Article12. Vlasov IA, Volkov AM. Dependence of heart weight on body weight in patients with cardiovascular diseases. Fiziol Cheloveka. 2004; 30:62–68.13. Gaffney L, Warren P, Wrona EA, Fisher MB, Freytes DO. Macrophages' role in tissue disease and regeneration. Results Probl Cell Differ. 2017; 62:245–271.
Article14. Lavine KJ, Epelman S, Uchida K, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA. 2014; 111:16029–16034.
Article15. Zhang JY, Yang Z, Fang K, Shi ZL, Ren DH, Sun J. Oleuropein prevents the development of experimental autoimmune myocarditis in rats. Int Immunopharmacol. 2017; 48:187–195.
Article16. Speranskii AI, Kostyuk SV, Kalashnikova EA, Veiko NN. Enrichment of extracellular DNA from the cultivation medium of human peripheral blood mononuclears with genomic CpG rich fragments results in increased cell production of IL-6 and TNF-a via activation of the NF-kB signaling pathway. Biomed Khim. 2016; 62:331–340.
Article17. Lee JC, Hou MF, Huang HW, et al. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013; 13:55.
Article18. Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007; 204:3037–3047.
Article19. Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013; 112:1624–1633.
Article20. Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006; 25:6680–6684.21. Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007; 8:49–62.22. Capece D, Verzella D, Tessitore A, Alesse E, Capalbo C, Zazzeroni F. Cancer secretome and inflammation: the bright and the dark sides of NF-κB. [Epub ahead of print]. Semin Cell Dev Biol. 2017.
Article23. Catrysse L, van Loo G. Inflammation and the metabolic syndrome: the tissue-specific functions of NF-κB. Trends Cell Biol. 2017; 27:417–429.
Article24. Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005; 312:1097–1105.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pan-Nox inhibitor treatment improves renal function in aging murine diabetic kidneys
- Investigation of the Expression of Fractalkine and the Infiltration Characteristics of Fractalkine Receptor Positive Cells and Macrophages in Cisplatin-induced Acute Renal Failure in Mice
- IDH2 gene deficiency accelerates unilateral ureteral obstructioninduced kidney inflammation through oxidative stress and activation of macrophages
- Retinoic acid ameliorates rheumatoid arthritis by attenuating inflammation and modulating macrophage polarization through MKP-1/MAPK signaling pathway
- Paeonol accelerates skin wound healing by regulating macrophage polarization and inflammation in diabetic rats