Korean J Physiol Pharmacol.
2021 Mar;25(2):139-146. 10.4196/kjpp.2021.25.2.139.
IDH2 gene deficiency accelerates unilateral ureteral obstructioninduced kidney inflammation through oxidative stress and activation of macrophages
- Affiliations
-
- 1Department of Molecular Medicine and Medical Research Center, Keimyung University School of Medicine, Daegu 42601, Korea
- 2Department of Anatomy and BK21 Plus, School of Medicine, Kyungpook National University, Daegu 41944, Korea
- KMID: 2512957
- DOI: http://doi.org/10.4196/kjpp.2021.25.2.139
Abstract
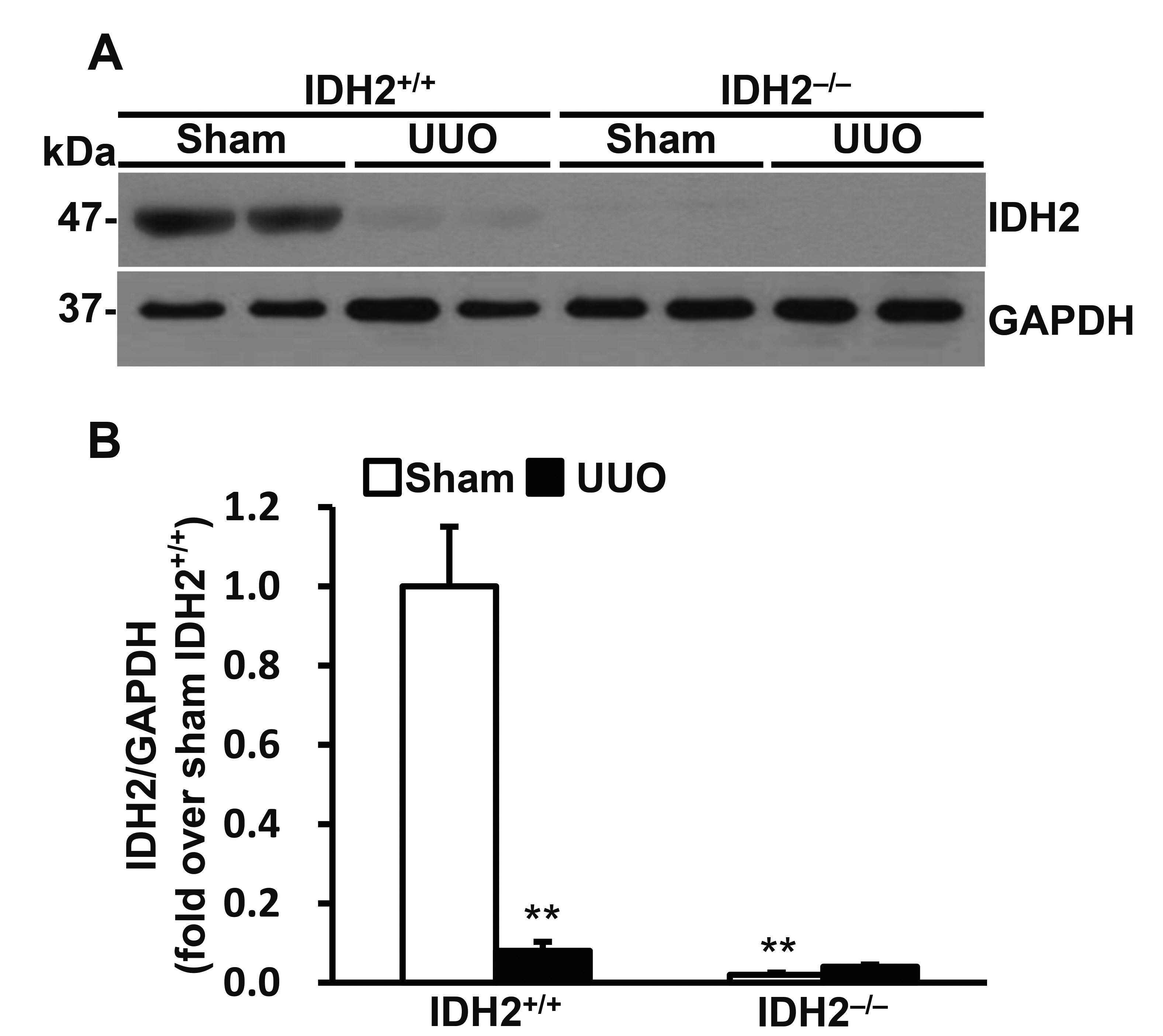
- Mitochondrial NADP+-dependent isocitrate dehydrogenase 2 (IDH2) produces NADPH, which is known to inhibit mitochondrial oxidative stress. Ureteral obstruction induces kidney inflammation and fibrosis via oxidative stress. Here, we investigated the role and underlying mechanism of IDH2 in unilateral ureteral obstruction (UUO)-induced kidney inflammation using IDH2 gene deleted mice (IDH2–/–). Eight- to 10-week-old female IDH2–/– mice and wild type (IDH2+/+) littermates were subjected to UUO and kidneys were harvested 5 days after UUO. IDH2 was not detected in the kidneys of IDH2–/– mice, while UUO decreased IDH2 in IDH2+/+ mice. UUO increased the expressions of markers of oxidative stress in both IDH2+/+ and IDH2–/– mice, and these changes were greater in IDH2–/– mice compared to IDH2+/+ mice. Bone marrow-derived macrophages of IDH2–/– mice showed a more migrating phenotype with greater ruffle formation and Rac1 distribution than that of IDH2+/+ mice. Correspondently, UUO-induced infiltration of monocytes/macrophages was greater in IDH2–/– mice compared to IDH2+/+ mice. Taken together, these data demonstrate that IDH2 plays a protective role against UUO-induced inflammation through inhibition of oxidative stress and macrophage infiltration.
Keyword
Figure
Reference
-
1. Sacks SH, Aparicio SA, Bevan A, Oliver DO, Will EJ, Davison AM. 1989; Late renal failure due to prostatic outflow obstruction: a preventable disease. BMJ. 298:156–159. DOI: 10.1136/bmj.298.6667.156. PMID: 2466506. PMCID: PMC1835497.
Article2. Chevalier RL, Thornhill BA, Forbes MS, Kiley SC. 2010; Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol. 25:687–697. DOI: 10.1007/s00467-009-1316-5. PMID: 19844747.
Article3. Ucero AC, Benito-Martin A, Izquierdo MC, Sanchez-Niño MD, Sanz AB, Ramos AM, Berzal S, Ruiz-Ortega M, Egido J, Ortiz A. 2014; Unilateral ureteral obstruction: beyond obstruction. Int Urol Nephrol. 46:765–776. DOI: 10.1007/s11255-013-0520-1. PMID: 24072452.
Article4. Dendooven A, Ishola DA Jr, Nguyen TQ, Van der Giezen DM, Kok RJ, Goldschmeding R, Joles JA. 2011; Oxidative stress in obstructive nephropathy. Int J Exp Pathol. 92:202–210. DOI: 10.1111/j.1365-2613.2010.00730.x. PMID: 20804541. PMCID: PMC3101492.
Article5. Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. 2009; Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 11:2685–2700. DOI: 10.1089/ars.2009.2695. PMID: 19558212. PMCID: PMC2821140.
Article6. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. 2012; Oxidative stress and antioxidant defense. World Allergy Organ J. 5:9–19. DOI: 10.1097/WOX.0b013e3182439613. PMID: 23268465. PMCID: PMC3488923.
Article7. Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC, Yung KY, Brenner D, Knobbe-Thomsen CB, Cox MA, Elia A, Berger T, Cescon DW, Adeoye A, Brüstle A, Molyneux SD, Mason JM, Li WY, Yamamoto K, Wakeham A, et al. 2015; Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 27:211–222. DOI: 10.1016/j.ccell.2014.11.019. PMID: 25620030.
Article8. Reitman ZJ, Yan H. 2010; Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 102:932–941. DOI: 10.1093/jnci/djq187. PMID: 20513808. PMCID: PMC2897878.
Article9. Lee SJ, Cha H, Lee S, Kim H, Ku HJ, Kim SH, Park JH, Lee JH, Park KM, Park JW. 2017; Idh2 deficiency accelerates renal dysfunction in aged mice. Biochem Biophys Res Commun. 493:34–39. DOI: 10.1016/j.bbrc.2017.09.082. PMID: 28928092.
Article10. Han SJ, Jang HS, Noh MR, Kim J, Kong MJ, Kim JI, Park JW, Park KM. 2017; Mitochondrial NADP+-dependent isocitrate dehydrogenase deficiency exacerbates mitochondrial and cell damage after kidney ischemia-reperfusion injury. J Am Soc Nephrol. 28:1200–1215. DOI: 10.1681/ASN.2016030349. PMID: 27821630. PMCID: PMC5373447.11. Han SJ, Choi HS, Kim JI, Park JW, Park KM. 2018; IDH2 deficiency increases the liver susceptibility to ischemia-reperfusion injury via increased mitochondrial oxidative injury. Redox Biol. 14:142–153. DOI: 10.1016/j.redox.2017.09.003. PMID: 28938192. PMCID: PMC5608561.
Article12. Kong MJ, Han SJ, Kim JI, Park JW, Park KM. 2018; Mitochondrial NADP+-dependent isocitrate dehydrogenase deficiency increases cisplatin-induced oxidative damage in the kidney tubule cells. Cell Death Dis. 9:488. DOI: 10.1038/s41419-018-0537-6. PMID: 29695796. PMCID: PMC5916920.
Article13. Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. 2004; Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol. 286:F606–F616. DOI: 10.1152/ajprenal.00269.2003. PMID: 15001451.
Article14. Eddy AA. 1995; Interstitial macrophages as mediators of renal fibrosis. Exp Nephrol. 3:76–79. PMID: 7773640.15. Nishida M, Hamaoka K. 2008; Macrophage phenotype and renal fibrosis in obstructive nephropathy. Nephron Exp Nephrol. 110:e31–e36. DOI: 10.1159/000151561. PMID: 18724069.
Article16. Pan JH, Kim HS, Beane KE, Montalbano AM, Lee JH, Kim YJ, Kim JH, Kong BC, Kim S, Park JW, Shin EC, Kim JK. 2018; IDH2 deficiency aggravates fructose-induced NAFLD by modulating hepatic fatty acid metabolism and activating inflammatory signaling in female mice. Nutrients. 10:679. DOI: 10.3390/nu10060679. PMID: 29861476. PMCID: PMC6024877.
Article17. Kim S, Kim SY, Ku HJ, Jeon YH, Lee HW, Lee J, Kwon TK, Park KM, Park JW. 2014; Suppression of tumorigenesis in mitochondrial NADP(+)-dependent isocitrate dehydrogenase knock-out mice. Biochim Biophys Acta. 1842:135–143. DOI: 10.1016/j.bbadis.2013.11.008. PMID: 24240089.
Article18. Kim JI, Noh MR, Kim KY, Jang HS, Kim HY, Park KM. 2015; Methionine sulfoxide reductase A deficiency exacerbates progression of kidney fibrosis induced by unilateral ureteral obstruction. Free Radic Biol Med. 89:201–208. DOI: 10.1016/j.freeradbiomed.2015.07.018. PMID: 26210777.
Article19. Shin AH, Kil IS, Yang ES, Huh TL, Yang CH, Park JW. 2004; Regulation of high glucose-induced apoptosis by mitochondrial NADP+-dependent isocitrate dehydrogenase. Biochem Biophys Res Commun. 325:32–38. DOI: 10.1016/j.bbrc.2004.09.218. PMID: 15522197.20. Frezza C, Cipolat S, Scorrano L. 2007; Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2:287–295. DOI: 10.1038/nprot.2006.478. PMID: 17406588.21. Noh MR, Jang HS, Song DK, Lee SR, Lipschutz JH, Park KM, Kim JI. 2018; Downregulation of exocyst Sec10 accelerates kidney tubule cell recovery through enhanced cell migration. Biochem Biophys Res Commun. 496:309–315. DOI: 10.1016/j.bbrc.2018.01.013. PMID: 29326040.
Article22. Jo SH, Son MK, Koh HJ, Lee SM, Song IH, Kim YO, Lee YS, Jeong KS, Kim WB, Park JW, Song BJ, Huh TL. 2001; Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem. 276:16168–16176. DOI: 10.1074/jbc.M010120200. PMID: 11278619.23. Klahr S, Morrissey J. 2002; Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 283:F861–F875. DOI: 10.1152/ajprenal.00362.2001. PMID: 12372761.
Article24. Yeh CH, Chiang HS, Lai TY, Chien CT. 2011; Unilateral ureteral obstruction evokes renal tubular apoptosis via the enhanced oxidative stress and endoplasmic reticulum stress in the rat. Neurourol Urodyn. 30:472–479. DOI: 10.1002/nau.20855. PMID: 21305585.
Article25. Kinter M, Wolstenholme JT, Thornhill BA, Newton EA, McCormick ML, Chevalier RL. 1999; Unilateral ureteral obstruction impairs renal antioxidant enzyme activation during sodium depletion. Kidney Int. 55:1327–1334. DOI: 10.1046/j.1523-1755.1999.00358.x. PMID: 10200997.
Article26. Sugiyama H, Kobayashi M, Wang DH, Sunami R, Maeshima Y, Yamasaki Y, Masuoka N, Kira S, Makino H. 2005; Telmisartan inhibits both oxidative stress and renal fibrosis after unilateral ureteral obstruction in acatalasemic mice. Nephrol Dial Transplant. 20:2670–2680. DOI: 10.1093/ndt/gfi045. PMID: 16141465.
Article27. Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. 2016; Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. 25:119–146. DOI: 10.1089/ars.2016.6665. PMID: 26906267. PMCID: PMC4948213.
Article28. Nishida M, Fujinaka H, Matsusaka T, Price J, Kon V, Fogo AB, Davidson JM, Linton MF, Fazio S, Homma T, Yoshida H, Ichikawa I. 2002; Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest. 110:1859–1868. DOI: 10.1172/JCI200215045. PMID: 12488436. PMCID: PMC151648.
Article29. Munnamalai V, Suter DM. 2009; Reactive oxygen species regulate F-actin dynamics in neuronal growth cones and neurite outgrowth. J Neurochem. 108:644–661. DOI: 10.1111/j.1471-4159.2008.05787.x. PMID: 19054285. PMCID: PMC2995541.
Article30. Sakai J, Li J, Subramanian KK, Mondal S, Bajrami B, Hattori H, Jia Y, Dickinson BC, Zhong J, Ye K, Chang CJ, Ho YS, Zhou J, Luo HR. 2012; Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity. 37:1037–1049. DOI: 10.1016/j.immuni.2012.08.017. PMID: 23159440. PMCID: PMC3525814.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The flavonoid fisetin ameliorates renal fibrosis by inhibiting SMAD3 phosphorylation, oxidative damage, and inflammation in ureteral obstructed kidney in mice
- TDAG51 deficiency promotes oxidative stress-induced apoptosis through the generation of reactive oxygen species in mouse embryonic fibroblasts
- The role of oxidative stress and hypoxia in renal disease
- The comparative effects of intravenous iron on oxidative stress and inflammation in patients with chronic kidney disease and iron deficiency: a randomized controlled pilot study
- Pathomechanism of oxidative stress in cardiovascularrenal remodeling and therapeutic strategies