Korean J Physiol Pharmacol.
2023 Sep;27(5):437-448. 10.4196/kjpp.2023.27.5.437.
Paeonol accelerates skin wound healing by regulating macrophage polarization and inflammation in diabetic rats
- Affiliations
-
- 1Department of Orthopedics, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, Hunan 410004, China
- KMID: 2545534
- DOI: http://doi.org/10.4196/kjpp.2023.27.5.437
Abstract
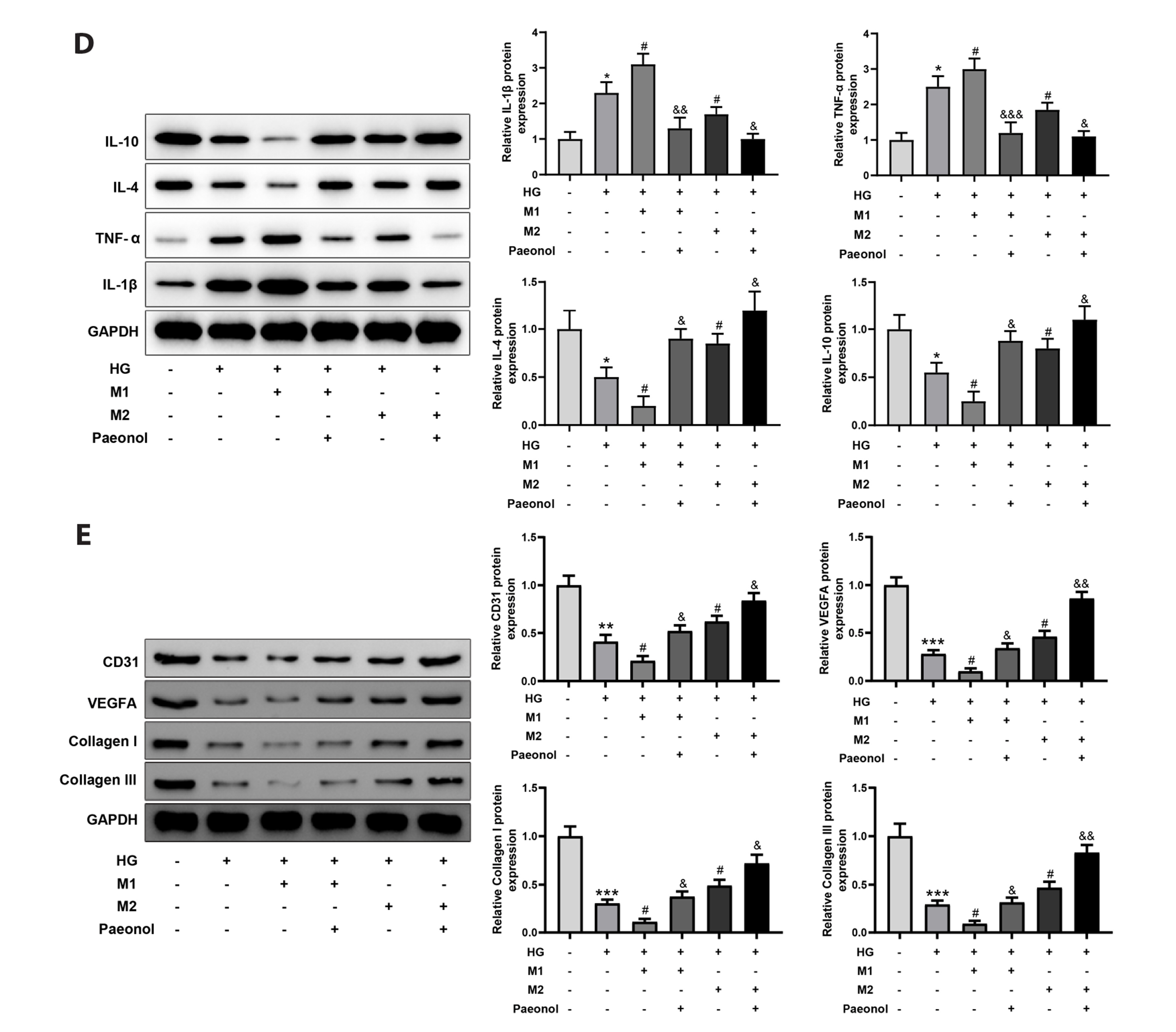
- Diabetic ulcer is usually seen in people with uncontrolled blood sugar. Reportedly, many factors such as impaired glucose metabolism, and macrovascular and microvascular diseases caused angiogenesis disorders and delayed the healing of diabetic ulcers, thus affecting the body's metabolism, nutrition, and immune function. This study aimed to explore the effect of paeonol on skin wound healing in diabetic rats and the related mechanism. A rat model of diabetic ulcer was established. High glucose-treated mouse skin fibroblasts were co-cultured with M1 or M2-polarized macrophages treated with or without paeonol. H&E and Masson staining were used to reveal inflammatory cell infiltration and collagen deposition, respectively. Immunohistochemistry visualized the expression of Ki67, CD31, and vascular endothelial growth factor (VEGF). Western blot was used to detect interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-4, IL-10, CD31, VEGFA, and collagen I/III. The expression of iNOS and arginase 1 was revealed by immunofluorescence staining. Paeonol treatment augmented collagen deposition and the expression of Ki67, CD31, VEGF, and macrophage M2 polarization markers (IL-4 and IL-10) and reduced wound area, inflammatory cell infiltration, and macrophage M1 polarization markers (IL-1β and TNF-α) in the ulcerated area. In vitro, paeonol treatment promoted M2-polarization and repressed M1-polarization in macrophages, thereby improving the repair of cell damage induced by high glucose. Paeonol accelerates the healing of diabetic ulcers by promoting M2 macrophage polarization and inhibiting M1 macrophage polarization.
Keyword
Figure
Reference
-
1. Petersmann A, Müller-Wieland D, Müller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, Schleicher E. 2019; Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 127:S1–S7. DOI: 10.1055/a-1018-9078. PMID: 31860923.
Article2. Schmidt AM. 2018; Highlighting diabetes mellitus: the epidemic continues. Arterioscler Thromb Vasc Biol. 38:e1–e8. DOI: 10.1161/ATVBAHA.117.310221. PMCID: PMC5776687.3. Zheng Y, Ley SH, Hu FB. 2018; Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 14:88–98. DOI: 10.1038/nrendo.2017.151. PMID: 29219149.
Article4. den Dekker A, Davis FM, Kunkel SL, Gallagher KA. 2019; Targeting epigenetic mechanisms in diabetic wound healing. Transl Res. 204:39–50. DOI: 10.1016/j.trsl.2018.10.001. PMID: 30392877. PMCID: PMC6331222.5. Okonkwo UA, DiPietro LA. 2017; Diabetes and wound angiogenesis. Int J Mol Sci. 18:1419. DOI: 10.3390/ijms18071419. PMID: 28671607. PMCID: PMC5535911. PMID: ddc8a47842c44935a237ca9dd22b845a.
Article6. Huang X, Liang P, Jiang B, Zhang P, Yu W, Duan M, Guo L, Cui X, Huang M, Huang X. 2020; Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 259:118246. DOI: 10.1016/j.lfs.2020.118246. PMID: 32791151.
Article7. Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. 2017; Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol. 199:17–24. DOI: 10.4049/jimmunol.1700223. PMID: 28630109.
Article8. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. 2018; Macrophage plasticity, polarization, and function in health and disease. . J Cell Physiol. 233:6425–6440. DOI: 10.1002/jcp.26429. PMID: 29319160.
Article9. Yunna C, Mengru H, Lei W, Weidong C. 2020; Macrophage M1/M2 polarization. Eur J Pharmacol. 877:173090. DOI: 10.1016/j.ejphar.2020.173090. PMID: 32234529.
Article10. Kim SY, Nair MG. 2019; Macrophages in wound healing: activation and plasticity. Immunol Cell Biol. 97:258–267. DOI: 10.1111/imcb.12236. PMID: 30746824. PMCID: PMC6426672.
Article11. Huang YY, Lin CW, Cheng NC, Cazzell SM, Chen HH, Huang KF, Tung KY, Huang HL, Lin PY, Perng CK, Shi B, Liu C, Ma Y, Cao Y, Li Y, Xue Y, Yan L, Li Q, Ning G, Chang SC. 2021; Effect of a novel macrophage-regulating drug on wound healing in patients with diabetic foot ulcers: a randomized clinical trial. JAMA Netw Open. 4:e2122607. DOI: 10.1001/jamanetworkopen.2021.22607. PMID: 34477854. PMCID: PMC8417758.12. Louiselle AE, Niemiec SM, Zgheib C, Liechty KW. 2021; Macrophage polarization and diabetic wound healing. Transl Res. 236:109–116. DOI: 10.1016/j.trsl.2021.05.006. PMID: 34089902.
Article13. Yang H, Song L, Sun B, Chu D, Yang L, Li M, Li H, Dai Y, Yu Z, Guo J. 2021; Modulation of macrophages by a paeoniflorin-loaded hyaluronic acid-based hydrogel promotes diabetic wound healing. Mater Today Bio. 12:100139. DOI: 10.1016/j.mtbio.2021.100139. PMID: 34632363. PMCID: PMC8488309.
Article14. Park YS, Uddin MJ, Piao L, Hwang I, Lee JH, Ha H. 2016; Novel role of endogenous catalase in macrophage polarization in adipose tissue. Mediators Inflamm. 2016:8675905. DOI: 10.1155/2016/8675905. PMID: 27597806. PMCID: PMC5002490. PMID: 59c79c8776b240ebabe0720dfba21fe3.
Article15. Zhang L, Li DC, Liu LF. 2019; Paeonol: pharmacological effects and mechanisms of action. Int Immunopharmacol. 72:413–421. DOI: 10.1016/j.intimp.2019.04.033. PMID: 31030097.
Article16. Mei L, He M, Zhang C, Miao J, Wen Q, Liu X, Xu Q, Ye S, Ye P, Huang H, Lin J, Zhou X, Zhao K, Chen D, Zhou J, Li C, Li H. 2019; Paeonol attenuates inflammation by targeting HMGB1 through upregulating miR-339-5p. Sci Rep. 9:19370. DOI: 10.1038/s41598-019-55980-4. PMID: 31852965. PMCID: PMC6920373.
Article17. Miao J, Zhong J, Lan J, Ye S, Ye P, Li S, You A, Chen X, Liu X, Li H. 2021; Paeonol attenuates inflammation by confining HMGB1 to the nucleus. J Cell Mol Med. 25:2885–2899. DOI: 10.1111/jcmm.16319. PMID: 33534963. PMCID: PMC7957162.
Article18. Wang R, Lechtenberg M, Sendker J, Petereit F, Deters A, Hensel A. 2013; Wound-healing plants from TCM: in vitro investigations on selected TCM plants and their influence on human dermal fibroblasts and keratinocytes. Fitoterapia. 84:308–317. DOI: 10.1016/j.fitote.2012.12.020. PMID: 23266731.
Article19. Sun L, Li J, Gao W, Shi M, Tang F, Fu X, Chen X. 2021; Coaxial nanofibrous scaffolds mimicking the extracellular matrix transition in the wound healing process promoting skin regeneration through enhancing immunomodulation. . J Mater Chem B. 9:1395–1405. DOI: 10.1039/D0TB01933J. PMID: 33462572.
Article20. Yang J, Chen Z, Pan D, Li H, Shen J. 2020; Umbilical cord-derived mesenchymal stem cell-derived exosomes combined Pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomedicine. 15:5911–5926. DOI: 10.2147/IJN.S249129. PMID: 32848396. PMCID: PMC7429232.21. Liu J, Feng L, Ma D, Zhang M, Gu J, Wang S, Fu Q, Song Y, Lan Z, Qu R, Ma S. 2013; Neuroprotective effect of paeonol on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. Neurosci Lett. 549:63–68. DOI: 10.1016/j.neulet.2013.06.002. PMID: 23791853.
Article22. Abu-Al-Basal MA. 2010; Healing potential of Rosmarinus officinalis L. on full-thickness excision cutaneous wounds in alloxan-induced-diabetic BALB/c mice. J Ethnopharmacol. 131:443–450. DOI: 10.1016/j.jep.2010.07.007. PMID: 20633625.
Article23. Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY. 2019; Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci Rep. 9:840. DOI: 10.1038/s41598-018-37215-0. PMID: 30696869. PMCID: PMC6351546.
Article24. Ma T, Huang X, Zheng H, Huang G, Li W, Liu X, Liang J, Cao Y, Hu Y, Huang Y. 2021; SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxid Med Cell Longev. 2021:9265016. DOI: 10.1155/2021/9265016. PMID: 34790288. PMCID: PMC8592716.
Article25. M de-Brito N, Duncan-Moretti J, C da-Costa H, Saldanha-Gama R, Paula-Neto HA, G Dorighello G, L Simões R, Barja-Fidalgo C. 2020; Aerobic glycolysis is a metabolic requirement to maintain the M2-like polarization of tumor-associated macrophages. Biochim Biophys Acta Mol Cell Res. 1867:118604. DOI: 10.1016/j.bbamcr.2019.118604. PMID: 31760090.26. Tang H, Li K, Zhang S, Lan H, Liang L, Huang C, Li T. 2021; Inhibitory effect of paeonol on apoptosis, oxidative stress, and inflammatory response in human umbilical vein endothelial cells induced by high glucose and palmitic acid induced through regulating SIRT1/FOXO3a/NF-κB pathway. J Interferon Cytokine Res. 41:111–124. DOI: 10.1089/jir.2019.0236. PMID: 33750217.
Article27. Patel S, ivastava S Sr, Singh MR, Singh D. 2019; Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 112:108615. DOI: 10.1016/j.biopha.2019.108615. PMID: 30784919.
Article28. Davis FM, Kimball A, Boniakowski A, Gallagher K. 2018; Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr Diab Rep. 18:2. DOI: 10.1007/s11892-018-0970-z. PMID: 29362914.
Article29. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, Liu F, Yang L. 2020; Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 11:259. DOI: 10.1186/s13287-020-01756-x. PMID: 32600435. PMCID: PMC7322868. PMID: b3aa492d26cc4be3a3d71fd98269201c.
Article30. Yu T, Gao M, Yang P, Liu D, Wang D, Song F, Zhang X, Liu Y. 2019; Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing. J Cell Physiol. 234:4217–4231. DOI: 10.1002/jcp.27185. PMID: 30132863.
Article31. Hao Y, Yang L, Liu Y, Ye Y, Wang J, Yu C, Yan H, Xing Y, Jia Z, Hu C, Zuo H, Li Y. 2021; mmu-miR-145a-5p accelerates diabetic wound healing by promoting macrophage polarization toward the M2 phenotype. Front Med (Lausanne). 8:775523. DOI: 10.3389/fmed.2021.775523. PMID: 34993211. PMCID: PMC8724056. PMID: 2cc16beeb0cc4c91a9c437642fe4de58.
Article32. Li S, Ding X, Zhang H, Ding Y, Tan Q. 2022; IL-25 improves diabetic wound healing through stimulating M2 macrophage polarization and fibroblast activation. Int Immunopharmacol. 106:108605. DOI: 10.1016/j.intimp.2022.108605. PMID: 35149293.
Article33. Yang Y, Zhang B, Yang Y, Peng B, Ye R. 2022; FOXM1 accelerates wound healing in diabetic foot ulcer by inducing M2 macrophage polarization through a mechanism involving SEMA3C/NRP2/Hedgehog signaling. Diabetes Res Clin Pract. 184:109121. DOI: 10.1016/j.diabres.2021.109121. PMID: 34742786.
Article34. Xu F, Xiao H, Liu R, Yang Y, Zhang M, Chen L, Chen Z, Liu P, Huang H. 2019; Paeonol ameliorates glucose and lipid metabolism in experimental diabetes by activating Akt. Front Pharmacol. 10:261. DOI: 10.3389/fphar.2019.00261. PMID: 30941042. PMCID: PMC6433795. PMID: 5b70892eb04343cb9978c7245ee65321.
Article35. Liu C, Han Y, Gu X, Li M, Du Y, Feng N, Li J, Zhang S, Maslov LN, Wang G, Pei J, Fu F, Ding M. 2021; Paeonol promotes Opa1-mediated mitochondrial fusion via activating the CK2α-Stat3 pathway in diabetic cardiomyopathy. Redox Biol. 46:102098. DOI: 10.1016/j.redox.2021.102098. PMID: 34418601. PMCID: PMC8385203.36. Zhang L, Chen Z, Gong W, Zou Y, Xu F, Chen L, Huang H. 2018; Paeonol ameliorates diabetic renal fibrosis through promoting the activation of the Nrf2/ARE pathway via up-regulating Sirt1. Front Pharmacol. 9:512. DOI: 10.3389/fphar.2018.00512. PMID: 29867511. PMCID: PMC5968333. PMID: 6e28db1a490941039a56e7565e610b6c.
Article37. Adki KM, Kulkarni YA. 2021; Neuroprotective effect of paeonol in streptozotocin-induced diabetes in rats. Life Sci. 271:119202. DOI: 10.1016/j.lfs.2021.119202. PMID: 33577853.
Article38. Chen G, Guo T, Yang L. 2022; Paeonol reduces IL-β production by inhibiting the activation of nucleotide oligomerization domain-like receptor protein-3 inflammasome and nuclear factor-κB in macrophages. Biochem Cell Biol. 100:28–36. DOI: 10.1139/bcb-2021-0255. PMID: 34784237.
Article39. Yuan C, Xu X, Wang N, Zhu Q, Zhang J, Gong W, Ding Y, Xiao W, Chen W, Lu G, Yao G, Pan J, Wu K. 2022; Paeonol protects against acute pancreatitis by inhibiting M1 macrophage polarization via the NLRP3 inflammasomes pathway. Biochem Biophys Res Commun. 600:35–43. DOI: 10.1016/j.bbrc.2022.02.019. PMID: 35182973.
Article40. Kelley N, Jeltema D, Duan Y, He Y. 2019; The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 20:3328. DOI: 10.3390/ijms20133328. PMID: 31284572. PMCID: PMC6651423. PMID: 87e19faab2044c80ae8b33e5104b5f74.
Article41. Liu D, Yang P, Gao M, Yu T, Shi Y, Zhang M, Yao M, Liu Y, Zhang X. 2019; NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin Sci (Lond). 133:565–582. DOI: 10.1042/CS20180600. PMID: 30626731.
Article42. Qing L, Fu J, Wu P, Zhou Z, Yu F, Tang J. 2019; Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am J Transl Res. 11:655–668. PMID: 30899369. PMCID: PMC6413292.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polysaccharides isolated from Phellinus gilvus enhances dermal wound healing in streptozotocin-induced diabetic rats
- The Experimantal Studey of Delayed Wound Healing on Full-Thickness Skin Defects in Diabetic Rats
- Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells
- Impaired Wound Healing in Diabetes Mellitus
- A Skin Fixation Method for Decreasing the Influence of Wound Contraction on Wound Healing in a Rat Model