Int J Stem Cells.
2022 May;15(2):164-172. 10.15283/ijsc21067.
Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Burn Rectification, Affiliated Hospital of Nantong University, Nantong, China
- KMID: 2529788
- DOI: http://doi.org/10.15283/ijsc21067
Abstract
- Background and Objectives
Despite advances in wound treatments, chronic diabetic wounds remain a significant medi-cal challenge. Exosomes from mesenchymal stem cells (MSCs) and small molecule activators of nuclear factor erythroid 2–related factor 2 (Nrf2) have emerged as potential therapies for nonhealing diabetic wounds. This study aimed to evaluate the effects of exosomes from bone marrow-derived MSCs (BMSCs) alone, or in combination with a small molecule activator of Nrf2 on diabetic wound healing.
Methods and Results
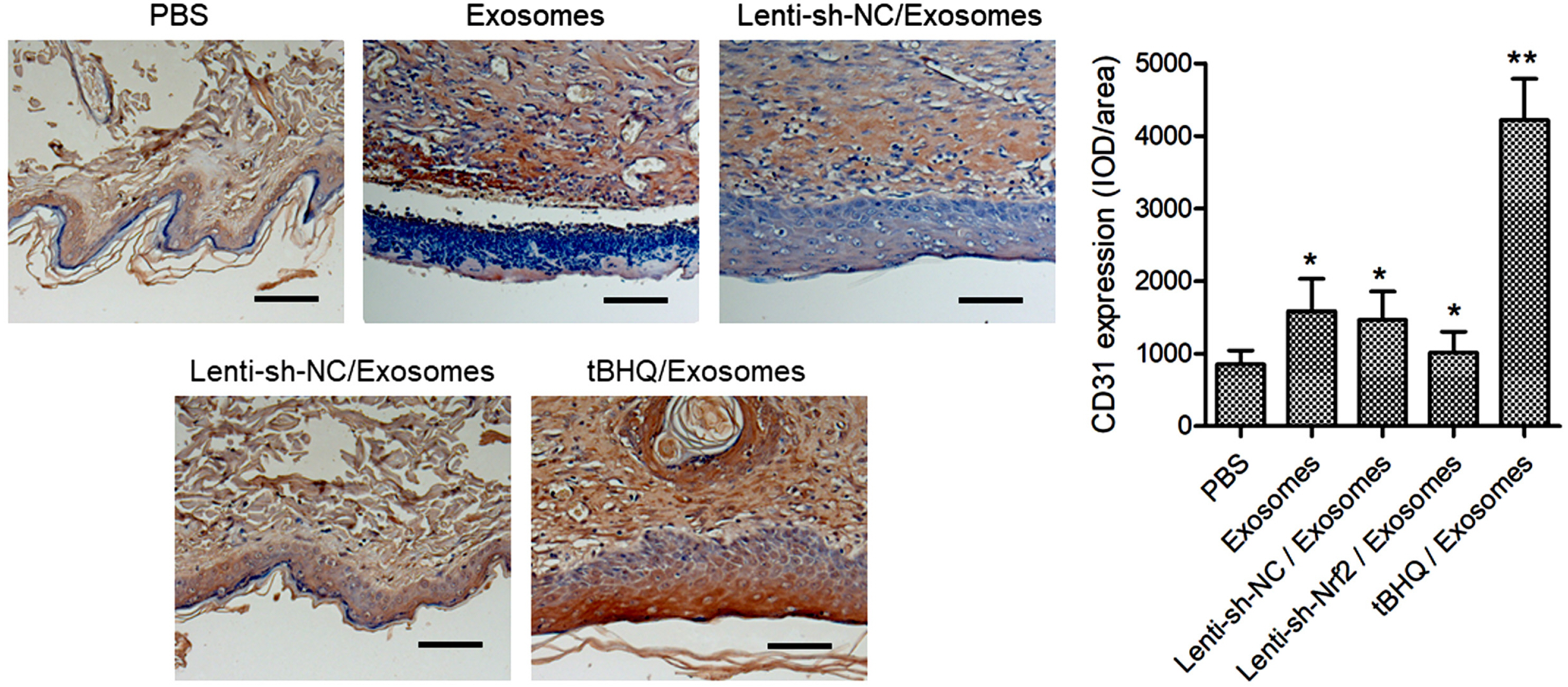
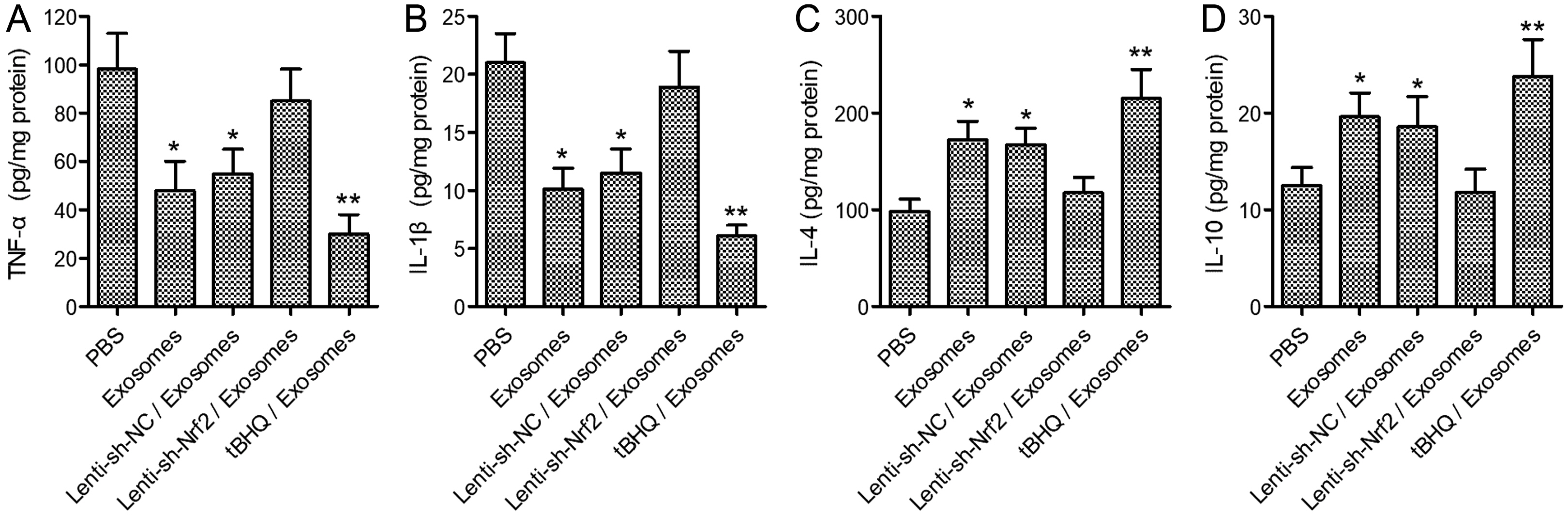
BMSCs and endothelial progenitor cells (EPCs) were isolated from the femur and tibia bone marrow of Sprague-Dawley (SD) rats and culture-expanded. Exosomes were harvested from the BMSC culture supernatants through ultracentrifugation. The effects of the exosomes and Nrf2 knockdown, alone or in combination, on EPC tube formation were evaluated. Streptozotocin-induced diabetic rats bearing a fresh full-thickness round wound were treated with the exosomes alone, or in combination with a lentiviral shRNA targeting Nrf2 (Lenti-sh-Nrf2) or tert-butylhydroquinone (tBHQ), a small molecule activator of Nrf2. Two weeks later, wound closure, re-epithelization, collagen deposition, neovascularization, and local inflammation were evaluated. BMSC exosomes promoted while Nrf2 knockdown inhibited EPC tube formation. BMSC exosomes accelerated wound closure, re-epithelization, collagen deposition, and neovascularization, and reduced wound inflammation in diabetic rats. These regenerative and anti-inflammatory effects of the exosomes were inhibited by Lenti-sh-Nrf2 but enhanced by tBHQ administration.
Conclusions
BMSC exosomes in combination with a small molecule Nrf2 activator hold promise as a new therapeutic option for chronic diabetic wounds.
Keyword
Figure
Reference
-
References
1. Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, Cartwright D. 2018; An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 21:27–32. DOI: 10.1016/j.jval.2017.07.007. PMID: 29304937.
Article2. Kosaric N, Kiwanuka H, Gurtner GC. 2019; Stem cell therapies for wound healing. Expert Opin Biol Ther. 19:575–585. DOI: 10.1080/14712598.2019.1596257. PMID: 30900481.
Article3. Hettich BF, Ben-Yehuda Greenwald M, Werner S, Leroux JC. 2020; Exosomes for wound healing: purification optimization and identification of bioactive components. Adv Sci (Weinh). 7:2002596. DOI: 10.1002/advs.202002596. PMID: 33304765. PMCID: PMC7709981.
Article4. Silva AM, Teixeira JH, Almeida MI, Gonçalves RM, Barbosa MA, Santos SG. 2017; Extracellular vesicles: immunomodulatory messengers in the context of tissue repair/regeneration. Eur J Pharm Sci. 98:86–95. DOI: 10.1016/j.ejps.2016.09.017. PMID: 27644894.
Article5. Hoang DH, Nguyen TD, Nguyen HP, Nguyen XH, Do PTX, Dang VD, Dam PTM, Bui HTH, Trinh MQ, Vu DM, Hoang NTM, Thanh LN, Than UTT. 2020; Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front Mol Biosci. 7:119. DOI: 10.3389/fmolb.2020.00119. PMID: 32671095. PMCID: PMC7327117. PMID: d8551c1647974a088970fa3aafd4b75b.
Article6. Ding J, Wang X, Chen B, Zhang J, Xu J. 2019; Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int. 2019:9742765. DOI: 10.1155/2019/9742765. PMID: 31192260. PMCID: PMC6525840.
Article7. Wu D, Kang L, Tian J, Wu Y, Liu J, Li Z, Wu X, Huang Y, Gao B, Wang H, Wu Z, Qiu G. 2020; Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe3O4 nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int J Nanomedicine. 15:7979–7993. DOI: 10.2147/IJN.S275650. PMID: 33116513. PMCID: PMC7585514.8. Wang C, Liang C, Wang R, Yao X, Guo P, Yuan W, Liu Y, Song Y, Li Z, Xie X. 2019; The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater Sci. 8:313–324. DOI: 10.1039/C9BM01207A. PMID: 31701966.
Article9. Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, Xu H, Lei B, Mao C. 2019; Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 9:65–76. DOI: 10.7150/thno.29766. PMID: 30662554. PMCID: PMC6332800.
Article10. He F, Ru X, Wen T. 2020; NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. 21:4777. DOI: 10.3390/ijms21134777. PMID: 32640524. PMCID: PMC7369905. PMID: 7528f37dbd2449179875581319f6f555.
Article11. Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R, Zhou S, Wong PK, Wondrak GT, Zheng H, Zhang DD. 2016; An essential role of NRF2 in diabetic wound healing. Diabetes. 65:780–793. DOI: 10.2337/db15-0564. PMID: 26718502. PMCID: PMC4764153.
Article12. Murasawa S, Asahara T. 2005; Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda). 20:36–42. DOI: 10.1152/physiol.00033.2004. PMID: 15653838.
Article13. Kaushik K, Das A. 2019; Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy. 21:1137–1150. DOI: 10.1016/j.jcyt.2019.09.002. PMID: 31668487.
Article14. Wang RY, Liu LH, Liu H, Wu KF, An J, Wang Q, Liu Y, Bai LJ, Qi BM, Qi BL, Zhang L. 2018; Nrf2 protects against diabetic dysfunction of endothelial progenitor cells via regulating cell senescence. Int J Mol Med. 42:1327–1340. DOI: 10.3892/ijmm.2018.3727. PMID: 29901179. PMCID: PMC6089760.
Article15. Fan J, Liu H, Wang J, Zeng J, Tan Y, Wang Y, Yu X, Li W, Wang P, Yang Z, Dai X. 2021; Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J Cell Mol Med. 25:652–665. DOI: 10.1111/jcmm.16111. PMID: 33215883. PMCID: PMC7812287.
Article16. Sun X, Wang X, Zhao Z, Chen J, Li C, Zhao G. 2020; Paeoniflorin accelerates foot wound healing in diabetic rats though activating the Nrf2 pathway. Acta Histochem. 122:151649. DOI: 10.1016/j.acthis.2020.151649. PMID: 33166863.
Article17. Li Y, Ma F, Li H, Song Y, Zhang H, Jiang Z, Wu H. 2018; Dimethyl fumarate accelerates wound healing under diabetic condition. J Mol Endocrinol. 61:163–172. DOI: 10.1530/JME-18-0102. PMID: 30038053.18. Gnecchi M, Melo LG. 2009; Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 482:281–294. DOI: 10.1007/978-1-59745-060-7_18. PMID: 19089363.
Article19. Chen Y, Ding H, Wei M, Zha W, Guan S, Liu N, Li Y, Tan Y, Wang Y, Wu F. 2020; MSC-secreted exosomal H19 promotes trophoblast cell invasion and migration by downregulating let-7b and upregulating FOXO1. Mol Ther Nucleic Acids. 19:1237–1249. DOI: 10.1016/j.omtn.2019.11.031. PMID: 32069774. PMCID: PMC7026285.
Article20. Zhang J, Zhang H, Chen Y, Fu J, Lei Y, Sun J, Tang B. 2019; Platelet‑derived growth factor D promotes the angiogenic capacity of endothelial progenitor cells. Mol Med Rep. 19:125–132. DOI: 10.3892/mmr.2018.9692. PMID: 30483778. PMCID: PMC6297765.21. Wang L, Wang F, Zhao L, Yang W, Wan X, Yue C, Mo Z. 2019; Mesenchymal stem cells coated by the extracellular matrix promote wound healing in diabetic rats. Stem Cells Int. 2019:9564869. DOI: 10.1155/2019/9564869. PMID: 30833970. PMCID: PMC6369500.
Article22. Zhao P, Sui BD, Liu N, Lv YJ, Zheng CX, Lu YB, Huang WT, Zhou CH, Chen J, Pang DL, Fei DD, Xuan K, Hu CH, Jin Y. 2017; Anti-aging pharmacology in cutaneous wound healing: effects of metformin, resveratrol, and rapamycin by local application. Aging Cell. 16:1083–1093. DOI: 10.1111/acel.12635. PMID: 28677234. PMCID: PMC5595695.
Article23. Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. 2014; MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 14:141–145. DOI: 10.1016/j.stem.2014.01.013. PMID: 24506881.
Article24. Shi Y, Kang X, Wang Y, Bian X, He G, Zhou M, Tang K. 2020; Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Monit. 26:e923328. DOI: 10.12659/MSM.923328. PMID: 32369458. PMCID: PMC7218969.
Article25. Werling NJ, Thorpe R, Zhao Y. 2013; A systematic approach to the establishment and characterization of endothelial progenitor cells for gene therapy. Hum Gene Ther Methods. 24:171–184. DOI: 10.1089/hgtb.2012.146. PMID: 23570242. PMCID: PMC3732128.
Article26. Li W, Kong AN. 2009; Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 48:91–104. DOI: 10.1002/mc.20465. PMID: 18618599. PMCID: PMC2631094.
Article27. Rekha PD, Rao SS, Sahana TG, Prabhu A. 2018; Diabetic wound management. Br J Community Nurs. 23(Sup9):S16–S22. DOI: 10.12968/bjcn.2018.23.Sup9.S16. PMID: 30156875.
Article28. Galipeau J, Sensébé L. 2018; Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 22:824–833. DOI: 10.1016/j.stem.2018.05.004. PMID: 29859173. PMCID: PMC6434696.
Article29. Yoon BS, Moon JH, Jun EK, Kim J, Maeng I, Kim JS, Lee JH, Baik CS, Kim A, Cho KS, Lee JH, Lee HH, Whang KY, You S. 2010; Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 19:887–902. DOI: 10.1089/scd.2009.0138. PMID: 19686050.
Article30. Cao Y, Gang X, Sun C, Wang G. 2017; Mesenchymal stem cells improve healing of diabetic foot ulcer. J Diabetes Res. 2017:9328347. DOI: 10.1155/2017/9328347. PMID: 28386568. PMCID: PMC5366201.
Article31. Fui LW, Lok MPW, Govindasamy V, Yong TK, Lek TK, Das AK. 2019; Understanding the multifaceted mechanisms of diabetic wound healing and therapeutic application of stem cells conditioned medium in the healing process. J Tissue Eng Regen Med. 13:2218–2233. DOI: 10.1002/term.2966. PMID: 31648415.
Article32. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, Liu F, Yang L. 2020; Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 11:259. DOI: 10.1186/s13287-020-01756-x. PMID: 32600435. PMCID: PMC7322868. PMID: b3aa492d26cc4be3a3d71fd98269201c.
Article33. Yu M, Liu W, Li J, Lu J, Lu H, Jia W, Liu F. 2020; Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 11:350. DOI: 10.1186/s13287-020-01824-2. PMID: 32787917. PMCID: PMC7425015. PMID: e88a65dcabb74898b0067962ac3f3f6c.
Article34. Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M. 2018; Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 50:1–14. DOI: 10.1038/s12276-018-0058-5. PMID: 29651102. PMCID: PMC5938041. PMID: 6991df1e4eaf487b80af28272104fed0.
Article35. Sharifzadeh G, Hosseinkhani H. 2017; Biomolecule-responsive hydrogels in medicine. Adv Healthc Mater. 6:1700801. DOI: 10.1002/adhm.201700801. PMID: 29057617.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exosomes Derived from Human Amniotic Mesenchymal Stem Cells Facilitate Diabetic Wound Healing by Angiogenesis and Enrich Multiple lncRNAs
- A Glimpse of Urine Stromal Cells-Derived Exosomes Containing Deleted in Malignant Brain Tumors 1: A Critical Factor in Wound Healing
- Comparison of Human Bone Marrow Stromal Cells with Fibroblasts in Cell Proliferation and Collagen Synthesis
- Human Adipose Mesenchymal Stem Cell-Derived Exosomes: A Key Player in Wound Healing
- Research Advances in the Application of Adipose-Derived Stem Cells Derived Exosomes in Cutaneous Wound Healing