Tuberc Respir Dis.
2018 Jan;81(1):80-87. 10.4046/trd.2017.0108.
Effects of Macrolide and Corticosteroid in Neutrophilic Asthma Mouse Model
- Affiliations
-
- 1Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. cmcyhg@gmail.com
- 2Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Anesthesiology and Pain Medicine, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2420594
- DOI: http://doi.org/10.4046/trd.2017.0108
Abstract
- BACKGROUND
Asthma is a disease of chronic airway inflammation with heterogeneous features. Neutrophilic asthma is corticosteroid-insensitive asthma related to absence or suppression of TH2 process and increased TH1 and/or TH17 process. Macrolides are immunomodulatory drug that reduce airway inflammation, but their role in asthma is not fully known. The purpose of this study was to evaluate the role of macrolides in neutrophilic asthma and compare their effects with those of corticosteroids.
METHODS
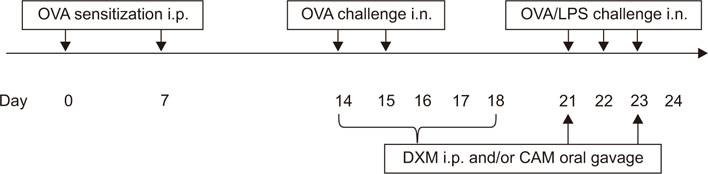
C57BL/6 female mice were sensitized with ovalbumin (OVA) and lipopolysaccharides (LPS). Clarithromycin (CAM) and/or dexamethasone (DXM) were administered at days 14, 15, 21, 22, and 23. At day 24, the mice were sacrificed.
RESULTS
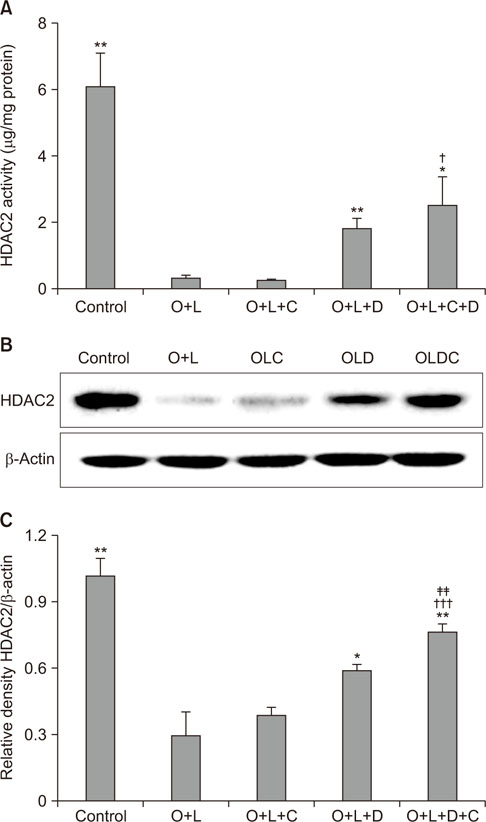
Airway resistance in the OVA+LPS exposed mice was elevated but was more attenuated after treatment with CAM+DXM compared with the monotherapy group (p < 0.05 and p < 0.01). In bronchoalveolar lavage fluid study, total cells and neutrophil counts in OVA+LPS mice were elevated but decreased after CAM+DXM treatment. In hematoxylin and eosin stain, the CAM+DXM-treated group showed less inflammation additively than the monotherapy group. There was less total protein, interleukin 17 (IL-17), interferon γ, and tumor necrosis factor α in the CAM+DXM group than in the monotherapy group (p < 0.001, p < 0.05, and p < 0.001). More histone deacetylase 2 (HDAC2) activity was recovered in the DXM and CAM+DXM challenged groups than in the control group (p < 0.05).
CONCLUSION
Decreased IL-17 and recovered relative HDAC2 activity correlated with airway resistance and inflammation in a neutrophilic asthma mouse model. This result suggests macrolides as a potential corticosteroid-sparing agent in neutrophilic asthma.
Keyword
MeSH Terms
-
Adrenal Cortex Hormones
Airway Resistance
Animals
Asthma*
Bronchoalveolar Lavage Fluid
Clarithromycin
Dexamethasone
Eosine Yellowish-(YS)
Female
Hematoxylin
Histone Deacetylase 2
Histone Deacetylases
Humans
Inflammation
Interferons
Interleukin-17
Lipopolysaccharides
Macrolides
Mice*
Neutrophils*
Ovalbumin
Th17 Cells
Tumor Necrosis Factor-alpha
Adrenal Cortex Hormones
Clarithromycin
Dexamethasone
Eosine Yellowish-(YS)
Hematoxylin
Histone Deacetylase 2
Histone Deacetylases
Interferons
Interleukin-17
Lipopolysaccharides
Macrolides
Ovalbumin
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Reddel HK, Hurd SS, FitzGerald JM. World Asthma Day. GINA 2014: a global asthma strategy for a global problem. Int J Tuberc Lung Dis. 2014; 18:505–506.
Article2. Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov. 2012; 11:958–972.
Article3. Wenzel SE. Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther. 2013; 26:710–715.
Article4. Barry LE, Sweeney J, O'Neill C, Price D, Heaney LG. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017; 18:129.
Article5. Accordini S, Corsico AG, Braggion M, Gerbase MW, Gislason D, Gulsvik A, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013; 160:93–101.
Article6. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380:2197–2223.7. Lai CK, Kim YY, Kuo SH, Spencer M, Williams AE. Cost of asthma in the Asia-Pacific region. Eur Respir Rev. 2006; 15:10–16.
Article8. Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009; 9:24.
Article9. Alam R, Good J, Rollins D, Verma M, Chu H, Pham TH, et al. Airway and serum biochemical correlates of refractory neutrophilic asthma. J Allergy Clin Immunol. 2017; 140:1004–1014.
Article10. Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002; 57:875–879.
Article11. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015; 16:45–56.
Article12. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 18:716–725.
Article13. Ito K, Herbert C, Siegle JS, Vuppusetty C, Hansbro N, Thomas PS, et al. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am J Respir Cell Mol Biol. 2008; 39:543–550.
Article14. Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010; 23:590–615.
Article15. Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacol Ther. 2008; 117:393–405.
Article16. Wong EH, Porter JD, Edwards MR, Johnston SL. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014; 2:657–670.
Article17. Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008; 177:148–155.
Article18. Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013; 68:322–329.
Article19. Bergquist M, Jonasson S, Hjoberg J, Hedenstierna G, Hanrieder J. Comprehensive multiplexed protein quantitation delineates eosinophilic and neutrophilic experimental asthma. BMC Pulm Med. 2014; 14:110.
Article20. Zhang F, Huang G, Hu B, Qian GS, Song Y. Recombinant HMGB1 A box protein inhibits Th17 responses in mice with neutrophilic asthma by suppressing dendritic cell-mediated Th17 polarization. Int Immunopharmacol. 2015; 24:110–118.
Article21. Zhao Y, Huang Y, He J, Li C, Deng W, Ran X, et al. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates airway inflammation by inhibiting the proliferation of effector T cells in a murine model of neutrophilic asthma. Immunol Lett. 2014; 157:9–15.22. Zhao S, Jiang Y, Yang X, Guo D, Wang Y, Wang J, et al. Lipopolysaccharides promote a shift from Th2-derived airway eosinophilic inflammation to Th17-derived neutrophilic inflammation in an ovalbumin-sensitized murine asthma model. J Asthma. 2017; 54:447–455.
Article23. McGovern TK, Robichaud A, Fereydoonzad L, Schuessler TF, Martin JG. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J Vis Exp. 2013; (75):e50172.
Article24. Shalaby KH, Gold LG, Schuessler TF, Martin JG, Robichaud A. Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness. Respir Res. 2010; 11:82.
Article25. Marwick JA, Ito K, Adcock IM, Kirkham PA. Oxidative stress and steroid resistance in asthma and COPD: pharmacological manipulation of HDAC-2 as a therapeutic strategy. Expert Opin Ther Targets. 2007; 11:745–755.
Article26. Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma: persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997; 156(3 Pt 1):737–743.27. Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999; 160(5 Pt 1):1532–1539.
Article28. Annunziato F, Romagnani S. Mouse T helper 17 phenotype: not so different than in man after all. Cytokine. 2011; 56:112–115.
Article29. Spagnolo P, Fabbri LM, Bush A. Long-term macrolide treatment for chronic respiratory disease. Eur Respir J. 2013; 42:239–251.
Article30. Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest. 2010; 138:1202–1212.
Article31. Kim RY, Pinkerton JW, Gibson PG, Cooper MA, Horvat JC, Hansbro PM. Inflammasomes in COPD and neutrophilic asthma. Thorax. 2015; 70:1199–1201.
Article32. Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013; 131:636–645.
Article33. Kobayashi Y, Wada H, Rossios C, Takagi D, Charron C, Barnes PJ, et al. A novel macrolide/fluoroketolide, solithromycin (CEM-101), reverses corticosteroid insensitivity via phosphoinositide 3-kinase pathway inhibition. Br J Pharmacol. 2013; 169:1024–1034.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum: Effects of Macrolide and Corticosteroid in Neutrophilic Asthma Mouse Model
- Tiotropium Bromide Has a More Potent Effect Than Corticosteroid in the Acute Neutrophilic Asthma Mouse Model
- Understanding asthma using animal models
- Tiotropium Bromide Improves Neutrophilic Asthma by Recovering Histone Deacetylase 2 Activity
- Neutrophilic asthma and Interleukin-8