J Korean Acad Conserv Dent.
2005 Sep;30(5):372-384.
MMP and TIMP production in periodontal ligament fibroblasts stimulated by Prevotella nigrescens lipopolysaccharide
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Seoul National University, Korea. hhson@snu.ac.kr
- 2Department of Conservative Dentistry, Asan Medical Center, Korea.
Abstract
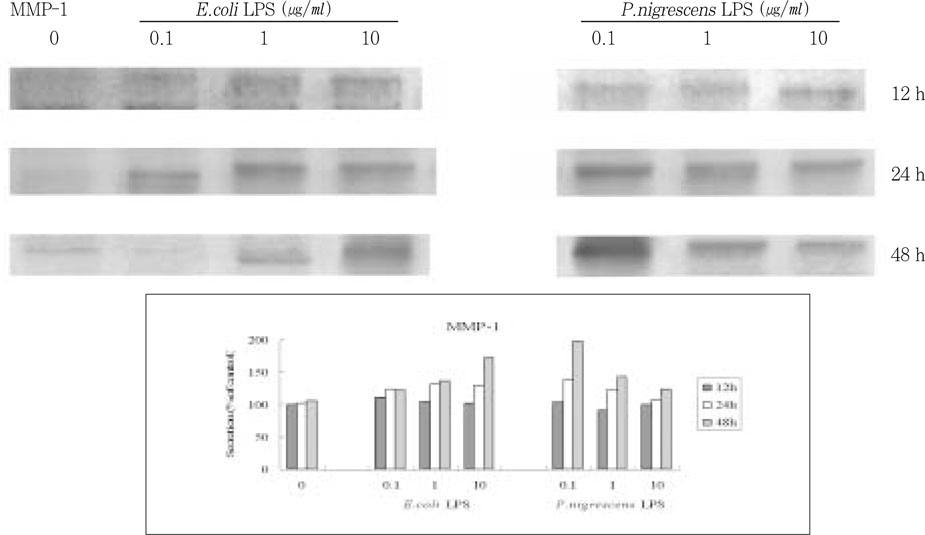
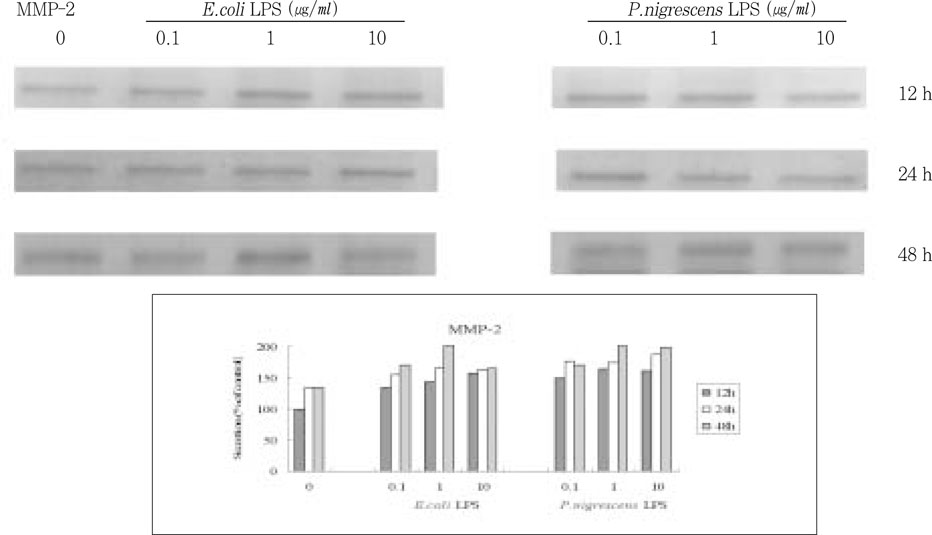
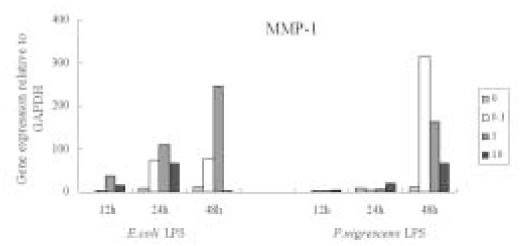
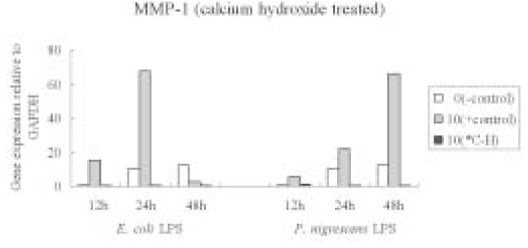
- The purpose of this study was to monitor the secretion of matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) by human periodontal ligament (PDL) fibroblasts stimulated with Prevotella nigrescens lipopolysaccharide (LPS), and to examine the effect of calcium hydroxide treatment on P. nigrescens LPS. LPS was extracted and purified from anaerobically cultured P. nigrescens. PDL fibroblasts were stimulated by the LPS (0, 0.1, 1, 10 ug/ml) or LPS (10 ug/ml) pretreated with 12.5 mg/ml of Ca(OH)2 for 3 days, for various periods of time (12, 24, 48 h). Immunoprecipitation were performed for protein level analysis of MMP-1, MMP-2 and TIMP-1. Total RNA was isolated and real-time quantitative polymerase chain reaction (PCR) was performed for quantification of MMP-1 mRNA. According to this study, the results were as follows: 1. The production of MMP-1 by stimulation with P. nigrescens LPS increased in time-dependent manner, and showed maximum value at 48 h in both protein and mRNA level. But there was no dose-dependent increase. 2. MMP-2 production time-dependently increased when stimulated with 1 and 10 ug/ml LPS, but there was no dose-dependent increase. 3. TIMP-1 production increased to 24 h, but decreased at 48 h. It increased when stimulated with 0.1 and 1 ug/ml LPS, but suppressed at 10 ug/ml. 4. P. nigrescens LPS pretreated with Ca(OH)2 markedly downregulated MMP-1 gene expression.
Keyword
MeSH Terms
Figure
Reference
-
1. Trowbridge HO, Stevens BH. Microbiologic and pathologic aspects of pulpal and periapical disease. Curr Opin Dent. 1992. 2:85–92.2. Yang YY, Tsai HF, Lu SC, Huang YF, Chang YC. Regulation of tissue inhibitors of metalloproteinase-1 gene expression by cytokines in human gingival fiboblasts. J Endod. 2002. 28(12):803–805.
Article3. Chang YCH, Lai CC, Yang SF, Chan Y, Hsieh YS. Stimulation of matrix metalloproteinases by black-pigmented bacteroides in human pulp and periodontal ligament cell cultures. J Endod. 2002. 28(2):90–93.
Article4. Lin SK, Wang CC, Huang S, Lee JJ, Chiang CP, Lan WH, Hong CY. Induction of dental pulp fibroblast matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 gene expression by interleukin-1α and tumor necrosis factor-α through a prostaglandin-dependent pathway. J Endod. 2001. 27(3):185–189.
Article5. Birkedal-Hansen H, Moore WBI, Bodden MK, Winsor LJ, Brikedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993. 4(2):197–250.
Article6. Douglas DA, Shi YE, Sang QA. Computational sequence analysis of the tissue inhibitor of metalloproteinase family. J Protein Chem. 1997. 16:237–255.7. Seguier S, Gogly B, Bodineau A, Godeau G, Brousse N. Is collagen breakdown during periodontitis linked to inflammatory cells and expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival tissue? J Periodontol. 2001. 72(10):1398–1406.
Article8. Reynolds JJ. Collagenases and tissue inhibitors of metalloproteinases: a functional balance in tissue degradation. Oral Dis. 1996. 2:70–76.
Article9. Reynolds JJ, Hembry RM, Meikle MC. Connective tissue degradation in health and periodontal disease and the roles of matrix metalloproteinases and their natural inhibitors. Adv Dent Res. 1994. 8(2):312–319.
Article10. Panagakos FA, O'Boskey FJ Jr, Rodriguez E. Regulation of pulp cell matrix metalloproteinase production by cytokines and lipopolysaccharides. J Endod. 1996. 22(7):358–361.
Article11. Tamura M, Nagaoka S, Kawagoe M. Interleukin-1α stimulates interstitial collagenase gene expression in human dental pulp fibroblast. J Endod. 1996. 22(5):240–243.
Article12. Hosoya S, Matsushima K. Stimulation of interleukin-1β production of human dental pulp cells by Porphyromonas endodontalis lipopolysaccharides. J Endod. 1997. 23(1):39–42.
Article13. Sundqvist G, Jahansson E, Sjogren U. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod. 1989. 15(1):13–19.
Article14. Baumgartner JC, Watkins BJ, Bae KS, Xia T. Association of black-pigmented bacteria with endodontic infections. J Endod. 1999. 25(6):413–415.
Article15. Siqueira JF, Rocas I, Oliveira JCM, Santos KRN. Molecular detection of black-pigmented bacteria in infections of endodontic origin. J Endod. 2001. 27(9):563–566.
Article16. Koga T, Nishihara T, Fujiwara T, Nisizawa T, Okahashi N, Noguchi T, Hamada S. Biochemical and immunological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun. 1985. 47(3):638–647.
Article17. Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Seydel U, Padova F, Schreier M, Brade H. Bacteial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994. 8:217–225.
Article18. Buck RA, Cai J, Eleazer PD, Staat RH, Hurst HE. Detoxification of endotoxin by endodontic irrigants and calcium hydroxide. J Endod. 2001. 27(5):325–327.
Article19. Safavi KE, Nichols FC. Effects of calcium hydroxide on bacterial lipopolysaccharide. J Endod. 1993. 19(2):76–78.20. Barthel CR, Levin LG, Reisner HM, Trope M. TNF-α release in monocytes after exposure to calcium hydroxide treated Escherichia coli LPS. Int Endod J. 1997. 30:155–159.
Article21. Cury JD, Campbell EJ, Lazarus CJ, Albin RJ, Welgus HG. Selective up-regulation of human alveolar macrophage collagenase production by lipopolysaccharide and comparison to collagenase production by fibroblasts. J Immunol. 1988. 141(12):4306–4312.22. Otsuka K, Sodek J, Limeback H. Synthesis of collagenase and collagenase inhibitors by osteoblast-like cells in culture. Eur J Biochem. 1984. 145(1):123–129.
Article23. Dougherty WJ, Bae KS, Watkins BJ, Baumgartner JC. Black-pigmented bacteria in coronal and apical segments of infected root canals. J Endod. 1998. 24(5):356–358.
Article24. Bae KS, Baumgartner JC, Shearer TR, David LL. Occurrence of Prevotella nigrescens and Prevotella intermedia in infections of endodontic origin. J Endod. 1997. 23:620–623.
Article25. Gharbia S, Haapasalo M, Shah H, et al. Characterization of Prevotella intermedia and Prevotella nigrescens isolates from periodontic and endodontic origin. J Periodontol. 1994. 65:56–61.
Article26. Firoozkoohi J, Zandi H, Olsen I. Comparison of lipopolysaccharides from Bacteroides, Porphylomonas, Prevotella, Camphylobacter and Wolinella spp. by Tricine-SDS-PAGE. Endod Dent Traumatol. 1997. 13:13–18.
Article27. Eidhin DN, Mouton C. A rapid method for preparation of rough and smooth lipopolysaccharides from Bacteroides, Porphylomonas and Prevotella. FEMS Microbiol Lett. 1993. 110:133–138.28. Oates TW, Rouse CA, Cochran DL. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J Periodontol. 1993. 64:142–148.
Article29. Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: Pitfalls and potential. Biotechniques. 1999. 26(1):112–125.
Article30. Gibson UEM, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996. 6:995–1001.
Article31. Shin SS, Lee JI, Baek SH, Lim SS. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endod. 2002. 28(4):313–315.
Article32. Chang YC, Yang SF, Hsieh YS. Regulation of matrix metalloproteinase-2 production by cytokines and pharmacological agents in human pulp cell cultures. J Endod. 2001. 27(11):679–682.
Article33. O'Boskey FJ Jr, Panagakos FS. Cytokines stimulate matrix metalloproteinase production by human pulp cells during long-term culture. J Endod. 1998. 24(1):7–10.34. Ueda I, Matsushima K. Stimulation of plasminogen activator activity and matrix metalloproteinases of human dental pulp-derived cells by tumor necrosis factor-α. J Endod. 2001. 27(3):175–179.
Article35. Nakata K, Yamasaki M, Iwata T, Suzuki K, Nakane A, Nakamura H. Anaerobic bacterial extracts influence production of matrix metalloproteinases and their inhibitors by human dental pulp cells. J Endod. 2000. 26(7):410–413.
Article36. Alvares O, Klebe R, Grant G, Cochran DL. Growth factor effects on the expression of collagenase and TIMP-1 in periodontal ligament cells. J Periodontol. 1995. 66:552–558.
Article37. Boyle DL, Rosengren S, Bugbee W, Kavanauph A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003. 5(6):R352–R360.38. Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF Jr. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989. 84:678–685.
Article39. Nakane A, Yoshida T, Nakata K, Horiba N, Nakamura H. Effects of lipopolysaccharides on human dental pulp cells. J Endod. 1995. 21(3):128–130.
Article40. Pinero G, Kiatpongsah S, Hutchins MO, Hoover J. The effect of endotoxin on the synthesis of connective tissue matrix components by pulp fibroblasts in vitro. J Endod. 1983. 9:2–7.
Article41. Schein B, Schilder H. Endotoxin content in endodontically involved teeth. J Endod. 1975. 1(1):19–21.
Article42. Horiba N, Maekawa Y, Abe Y, Ito M, Matsumoto T, Nakamura H. Correlations between endotoxin and clinical symptoms or radiolucent areas in infected root canals. Oral Surg Oral Med Oral Pathol. 1991. 71:492–495.
Article43. Yamasaki M, Nakane A, Kumazawa M, Hashioka K, Horiba N, Nakaura H. Endotoxin and gram-negative bacteria in the rat periapical lesions. J Endod. 1992. 18:501–504.
Article44. Safavi KE, Nichols FC. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J Endod. 1994. 20(3):127–129.
Article45. Nair BC, Mayberry WR, Dzaiak R, Chen PP, Levine MJ, Hausmann E. Biological effects of purified lipopolysaccharide from Bacteroides gingivalis. J Periodontal Res. 1983. 18:40–49.
Article46. Jun KC, Barua PK, Zambon JJ, Neiders ME. Proteolytic activity in black-pigmented bacteroides species. J Endod. 1989. 15(10):463–467.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of mRNA for matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival and periodontal ligament fibroblasts treated with lipopolysaccharide from Prevotella intermedia
- MMP-1 and TIMP-1 production in MG-63 cells stimulated with Prevotella nigrescens lipopolysaccharide
- Interleukin-8 production and interleukin-8 mRNA expression induced by lipopolysaccharides from Prevotella intermedia and Prevotella nigrescens in monocyte-derived macrophages
- Chemical and Immunobiological Characterization of Lipopolysaccharides from Prevotella intermedia and Prevotella nigrescens
- Effects of H2O2 and chlorhexidine on MMP-1, TIMP-1,2, Type 1 collagen, fibronectin and UNCL expressions in human periodontal ligament fibroblasts