J Korean Acad Periodontol.
2009 Jun;39(2):177-184. 10.5051/jkape.2009.39.2.177.
Interleukin-8 production and interleukin-8 mRNA expression induced by lipopolysaccharides from Prevotella intermedia and Prevotella nigrescens in monocyte-derived macrophages
- Affiliations
-
- 1Department of Periodontology, College of Dentistry, Pusan National University, Korea. sungjokim@pusan.ac.kr
- KMID: 1733735
- DOI: http://doi.org/10.5051/jkape.2009.39.2.177
Abstract
-
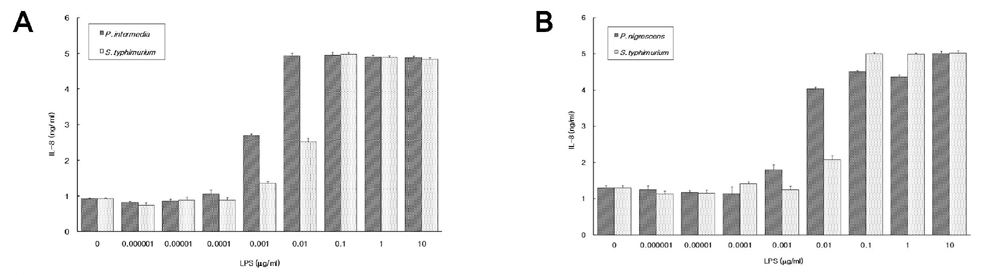
PURPOSE: Interleukin-8 (IL-8) is an important mediator of immune and inflammatory reactions and is produced by a variety of different cell types. This study was undertaken to investigate the effects of lipopolysaccharides (LPSs) from Prevotella intermedia and Prevotella nigrescens, the major causes of inflammatory periodontal disease, on the production of IL-8 and the expression of IL-8 mRNA in differentiated THP-1 cells, a human monocytic cell line.
METHODS
LPSs from P. intermedia ATCC 25611 and P. nigrescens ATCC 33563 were prepared by the standard hot phenol-water method. THP-1 cells were incubated in the medium supplemented with phorbol myristate acetate to induce differentiation into macrophage-like cells.
RESULTS
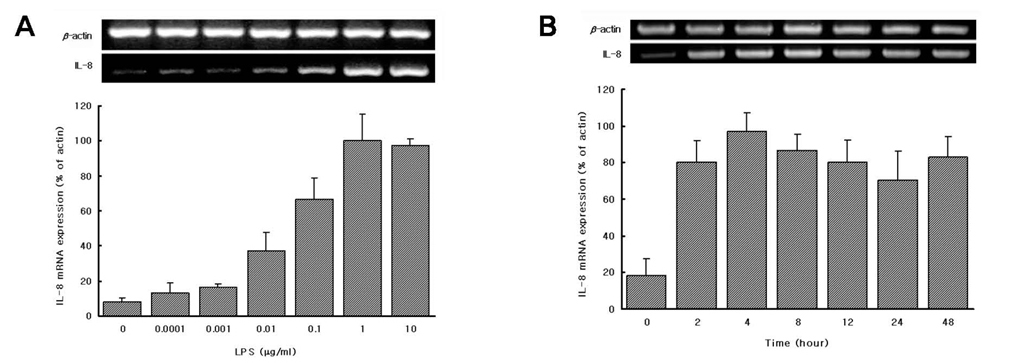
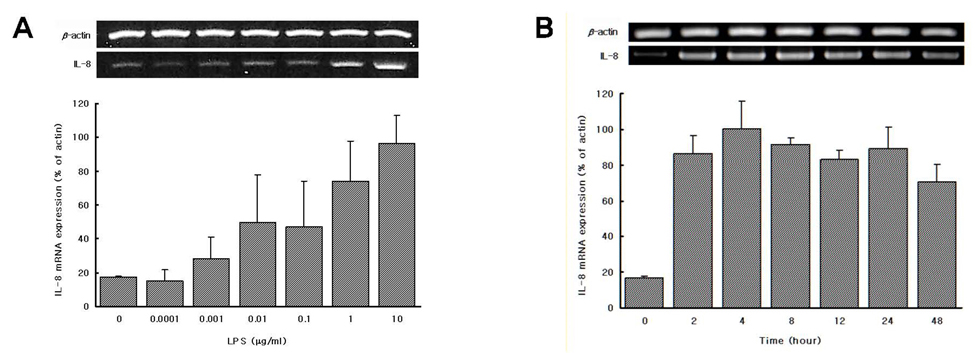
We found that LPS preparations from P. intermedia and P. nigrescens can induce IL-8 mRNA expression and stimulate the release of IL-8 in differentiated THP-1 cells without additional stimuli.
CONCLUSIONS
There are no previous reports of the ability of P. intermedia and P. nigrescens LPS to stimulate the release of IL-8, and the present study clearly shows, for the first time, that LPSs from P. intermedia and P. nigrescens fully induced IL-8 mRNA expression and IL-8 production in differentiated human monocytic cell line THP-1. The ability of P. intermedia and P. nigrescens LPS to promote the production of IL-8 may be important in the pathogenesis of inflammatory periodontal disease.
MeSH Terms
Figure
Reference
-
1. Zambon JJ. Periodontal diseases: microbial factor. Ann Periodontol. 1996. 1:879–925.2. Slots J, Bragd L, Wikstrom M, Dahlen G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986. 13:570–577.
Article3. Tanner ACR, Haffer C, Bratthall GT, Visconti RA, Socransky SS. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979. 6:278–307.
Article4. Chung CP, Nisengard RJ, Slots J, Genco RJ. Bacterial IgG and IgM antibody titers in acute necrotizing ulcerative gingivitis. J Periodontol. 1983. 54:557–562.
Article5. Kornman KS, Loesche WJ. The subgingival microbial flora during pregnancy. J Periodont Res. 1980. 15:111–122.
Article6. Lie MA, van der Weijden GA, Timmerman MF, Loos BG, van Steenbergen TJM, van der Velden U. Occurrence of Prevotella intermedia and Prevotella nigrescens in relation to gingival health. J Clin Periodontol. 2001. 28:189–193.
Article7. Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Microbial composition of supra and subgingival plaque in subjects with adult periodontitis. J Clin Periodontol. 2000. 27:722–732.
Article8. Teanpaisan R, Douglas CW, Walsh TF. Characterization of black-pigmented anaerobes isolated from diseased and healthy periodontal sites. J Periodont Res. 1995. 30:245–251.
Article9. Bae KS, Baumgartner JC, Shearer TR, David LL. Occurrence of Prevotella nigrescens and Prevotella intermedia in infections of endodontic origin. J Endod. 1997. 23:620–623.
Article10. Salcetti JM, Moriarty JD, Cooper LF, et al. The clinical, microbial, and host response characteristics of the failing implant. Int J Oral Maxillofac Implants. 1997. 12:32–42.11. Simon BI, Goldman HM, Ruben MP, Baker E. The role of endotoxin in periodontal disease. I. A reproducible, quantitative method for determining the amount of endotoxin in human gingival exudate. J Periodontol. 1969. 40:695–701.
Article12. Shapiro L, Lodato FM Jr, Courant PR, Stallard RE. Endotoxin determination in gingival inflammation. J Periodontol. 1972. 43:591–596.13. Maidwell-Smith MA, Wilson M, Kieser JB. Lipopolysaccharide (endotoxin) from periodontally involved teeth. J Clin Periodontol. 1987. 14:453–456.
Article14. Morrison DC, Ryan JL. Endotoxins and disease mechanisms. Annu Rev Med. 1987. 38:417–432.
Article15. Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000. 18:217–242.
Article16. Baggiolini M. Chemokines in pathology and medicine. J Int Med. 2001. 250:91–104.
Article17. Wuyts A, Proost P, Damme J. Thomson A, editor. Interleukin-8 and other CXC chemokines. The cytokine handbook. 1988. 3rd ed. London: Academic Press;Chapter 10.18. Thelen M, Peveri P, Kernen P, von Tscharaner V, Walz A, Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988. 2:2702–2706.
Article19. Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988. 167:1547–1559.
Article20. Detmers PA, Lo SK, Olsen EE, et al. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990. 171:1155–1162.
Article21. Westphal O, Jann K. Whistler RL, editor. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods in carbohydrate chemistry. 1965. New York: Academic Press;83–91.22. Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978. 87:206–210.
Article23. Hamada S, Takada H, Ogawa T, Fujiwara T, Mihara J. Lipopolysaccharides of oral anaerobes associated with chronic inflammation: chemical and immunomodulating properties. Int Rev Immunol. 1990. 6:247–261.
Article24. Kirikae T, Nitta T, Kirikae F, Suda Y, Kusumoto S, Qureshi N, Nakano M. Lipopolysaccharides (LPS) of oral black-pigmented bacteria induce tumor necrosis factor production by LPS-refractory C3H/HeJ macrophages in a way different from that of Salmonella LPS. Infect Immun. 1999. 67:1736–1742.
Article25. Fitzgerald JE, Kreutzer DL. Localization of interleukin-8 in human gingival tissues. Oral Microbiol Immunol. 1995. 10:297–303.
Article26. Tonetti MS, Imboden MA, Gerber L, Lang NP, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994. 62:4005–4014.
Article27. Tamura M, Tokuda M, Nagaoka S, Takada H. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infect Immun. 1992. 60:4932–4937.
Article28. Darveau RP, Belton CM, Reife RA, Lamont RJ. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun. 1998. 66:1660–1665.
Article29. Madianos PN, Papapanou PN, Sandros J. Porphyromonas gingivalis infection of oral eputhelium inhibits neutrophil transepithelial migration. Infect Immun. 1997. 65:3983–3990.
Article30. Watanabe A, Takeshita A, Kitano S, Hanazawa S. CD14-mediated signal pathway of Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Infect Immun. 1996. 64:4488–4494.
Article31. Shah HN, Gharbia SE. Proposal of a new species Prevotella nigrescens sp. nov. among strains previously classified as P. intermedia. FEMS Immunol Med Microbiol. 1993. 6:97.
Article32. Okamoto M, Maeda N, Kondo K, Leung KP. Hemolytic and hemagglutinating activities of Prevotella intermedia and Prevotella nigrescens. FEMS Microbiol Lett. 1999. 178:299–304.
Article33. Matto J, Asikainen S, Vaisanen ML, et al. Beta-lactamase production in Prevotella intermedia, Prevotella nigrescens, and Prevotella pallens genotypes and in vitro susceptibilities to selected antimicrobial agents. Antimicrob Agents Chemother. 1999. 43:2383–2388.
Article34. Andres MT, Chung WO, Roberts MC, Fierro JF. Antimicrobial susceptibilities of Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens spp. isolated in Spain. Antimicrob Agents Chemother. 1998. 42:3022–3023.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemical and Immunobiological Characterization of Lipopolysaccharides from Prevotella intermedia and Prevotella nigrescens
- Leptin potentiates Prevotella intermedia lipopolysaccharide-induced production of TNF-alpha in monocyte-derived macrophages
- Isolation and Partial Characterization of Hemin-binding Cell Envelope Proteins from Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens
- Curcumin suppresses the production of interleukin-6 in Prevotella intermedia lipopolysaccharide-activated RAW 264.7 cells
- Suppression of nitric oxide and interleukin-6 production by methanol extract of Sophorae Flos in macrophage cells