J Periodontal Implant Sci.
2011 Jun;41(3):157-163. 10.5051/jpis.2011.41.3.157.
Curcumin suppresses the production of interleukin-6 in Prevotella intermedia lipopolysaccharide-activated RAW 264.7 cells
- Affiliations
-
- 1Department of Periodontology, Pusan National University School of Dentistry, Yangsan, Korea. sungjokim@pusan.ac.kr
- KMID: 1783607
- DOI: http://doi.org/10.5051/jpis.2011.41.3.157
Abstract
- PURPOSE
Curcumin is known to exert numerous biological effects including anti-inflammatory activity. In this study, we investigated the effects of curcumin on the production of interleukin-6 (IL-6) by murine macrophage-like RAW 264.7 cells stimulated with lipopolysaccharide (LPS) from Prevotella intermedia, a major cause of inflammatory periodontal disease, and sought to determine the underlying mechanisms of action.
METHODS
LPS was prepared from lyophilized P. intermedia ATCC 25611 cells by the standard hot phenol-water method. Culture supernatants were collected and assayed for IL-6. We used real-time polymerase chain reaction to detect IL-6 mRNA expression. IkappaB-alpha degradation, nuclear translocation of NF-kappaB subunits, and STAT1 phosphorylation were characterized via immunoblotting. DNA-binding of NF-kappaB was also analyzed.
RESULTS
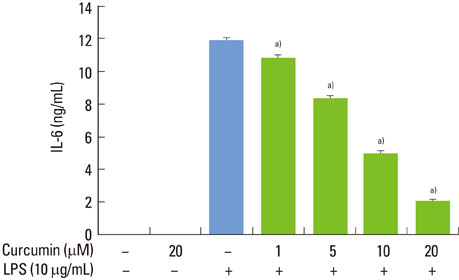
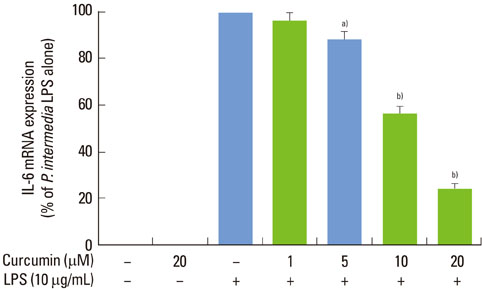
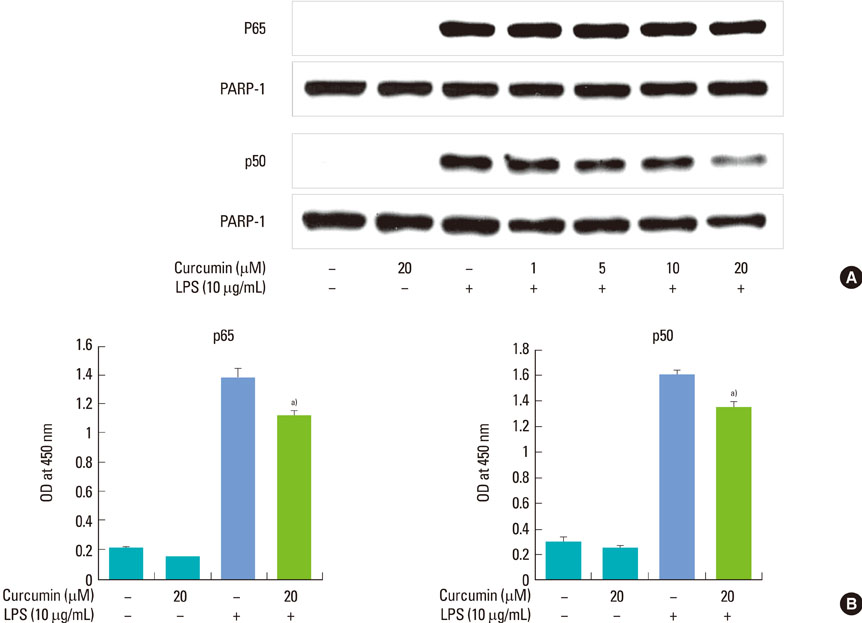
Curcumin strongly suppressed the production of IL-6 at both gene transcription and translation levels in P. intermedia LPS-activated RAW 264.7 cells. Curcumin did not inhibit the degradation of IkappaB-alpha induced by P. intermedia LPS. Curcumin blocked NF-kappaB signaling through the inhibition of nuclear translocation of NF-kappaB p50 subunit. Curcumin also attenuated DNA binding activity of p50 and p65 subunits and suppressed STAT1 phosphorylation.
CONCLUSIONS
Although further study is required to explore the detailed mechanism of action, curcumin may contribute to blockade of the host-destructive processes mediated by IL-6 and appears to have potential therapeutic values in the treatment of inflammatory periodontal disease.
MeSH Terms
Figure
Reference
-
1. Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999. 428:305–327.
Article2. Huang HC, Jan TR, Yeh SF. Inhibitory effect of curcumin, an anti-inflammatory agent, on vascular smooth muscle cell proliferation. Eur J Pharmacol. 1992. 221:381–384.
Article3. Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim Biophys Acta. 1994. 1224:255–263.
Article4. Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995. 94:79–83.
Article5. Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995. 55:259–266.6. Williams RC. Periodontal disease. N Engl J Med. 1990. 322:373–382.
Article7. Slots J, Bragd L, Wikström M, Dahlén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986. 13:570–577.
Article8. Tanner AC, Haffer C, Bratthall GT, Visconti RA, Socransky SS. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979. 6:278–307.
Article9. Chung CP, Nisengard RJ, Slots J, Genco RJ. Bacterial IgG and IgM antibody titers in acute necrotizing ulcerative gingivitis. J Periodontol. 1983. 54:557–562.
Article10. Kornman KS, Loesche WJ. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980. 15:111–122.
Article11. Morrison DC, Ryan JL. Endotoxins and disease mechanisms. Annu Rev Med. 1987. 38:417–432.
Article12. Hamada S, Takada H, Ogawa T, Fujiwara T, Mihara J. Lipopolysaccharides of oral anaerobes associated with chronic inflammation: chemical and immunomodulating properties. Int Rev Immunol. 1990. 6:247–261.
Article13. Kirikae T, Nitta T, Kirikae F, Suda Y, Kusumoto S, Qureshi N, et al. Lipopolysaccharides (LPS) of oral black-pigmented bacteria induce tumor necrosis factor production by LPS-refractory C3H/HeJ macrophages in a way different from that of Salmonella LPS. Infect Immun. 1999. 67:1736–1742.
Article14. Hashimoto M, Asai Y, Tamai R, Jinno T, Umatani K, Ogawa T. Chemical structure and immunobiological activity of lipid A from Prevotella intermedia ATCC 25611 lipopolysaccharide. FEBS Lett. 2003. 543:98–102.
Article15. Preshaw PM. Host response modulation in periodontics. Periodontol 2000. 2008. 48:92–110.
Article16. Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol. 2003. 8:12–37.
Article17. Kim SJ, Ha MS, Choi EY, Choi JI, Choi IS. Prevotella intermedia lipopolysaccharide stimulates release of nitric oxide by inducing expression of inducible nitric oxide synthase. J Periodontal Res. 2004. 39:424–431.
Article18. Kim SJ, Ha MS, Choi EY, Choi JI, Choi IS. Nitric oxide production and inducible nitric oxide synthase expression induced by Prevotella nigrescens lipopolysaccharide. FEMS Immunol Med Microbiol. 2005. 43:51–58.
Article19. Kim SJ, Choi EY, Kim EG, Shin SH, Lee JY, Choi JI, et al. Prevotella intermedia lipopolysaccharide stimulates release of tumor necrosis factor-alpha through mitogen-activated protein kinase signaling pathways in monocyte-derived macrophages. FEMS Immunol Med Microbiol. 2007. 51:407–413.
Article20. Choi EY, Jin JY, Lee JY, Choi JI, Choi IS, Kim SJ. Melatonin inhibits Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-6 in murine macrophages by suppressing NF-κB and STAT1 activity. J Pineal Res. 2011. 50:197–206.
Article21. Geivelis M, Turner DW, Pederson ED, Lamberts BL. Measurements of interleukin-6 in gingival crevicular fluid from adults with destructive periodontal disease. J Periodontol. 1993. 64:980–983.
Article22. Mogi M, Otogoto J, Ota N, Inagaki H, Minami M, Kojima K. Interleukin 1 beta, interleukin 6, beta 2-microglobulin, and transforming growth factor-alpha in gingival crevicular fluid from human periodontal disease. Arch Oral Biol. 1999. 44:535–539.
Article23. Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci U S A. 1993. 90:11924–11928.
Article24. Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005. 146:1991–1998.
Article25. Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994. 12:141–179.26. Rothwarf DM, Karin M. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE. 1999. 1999:RE1.27. Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000. 18:621–663.28. Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005. 309:1854–1857.
Article29. Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002. 2:725–734.30. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998. 16:225–260.
Article31. Pfitzner E, Kliem S, Baus D, Litterst CM. The role of STATs in inflammation and inflammatory diseases. Curr Pharm Des. 2004. 10:2839–2850.
Article32. Schindler C, Darnell JE Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995. 64:621–651.
Article33. Yamaoka K, Saharinen P, Pesu M, Holt VE 3rd, Silvennoinen O, O'Shea JJ. The Janus kinases (Jaks). Genome Biol. 2004. 5:253.
Article34. Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007. 282:20059–20063.
Article35. Gao JJ, Filla MB, Fultz MJ, Vogel SN, Russell SW, Murphy WJ. Autocrine/paracrine IFN-alphabeta mediates the lipopolysaccharide-induced activation of transcription factor Stat1alpha in mouse macrophages: pivotal role of Stat1alpha in induction of the inducible nitric oxide synthase gene. J Immunol. 1998. 161:4803–4810.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interleukin-8 production and interleukin-8 mRNA expression induced by lipopolysaccharides from Prevotella intermedia and Prevotella nigrescens in monocyte-derived macrophages
- Leptin potentiates Prevotella intermedia lipopolysaccharide-induced production of TNF-alpha in monocyte-derived macrophages
- Chemical and Immunobiological Characterization of Lipopolysaccharides from Prevotella intermedia and Prevotella nigrescens
- Effect of quercetin on the production of nitric oxide in murine macrophages stimulated with lipopolysaccharide from Prevotella intermedia
- Influence of the Sonic Power Toothbrush on Reduction of Gingival inflammation and on the Amount of Interleukin-6, Prevotella intermedia and Actinobacillus actinomycetemcomitans in Periodontal Pocket