J Rheum Dis.
2018 Jul;25(3):188-196. 10.4078/jrd.2018.25.3.188.
Compound K Inhibits Interleukin-1β-induced Expression of Inflammatory Mediators and Matrix Metalloproteinases by Inhibiting Mitogen-activated Protein Kinase Activation in Chondrocytes
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. elee@snu.ac.kr
- 2Division of Rheumatology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2415607
- DOI: http://doi.org/10.4078/jrd.2018.25.3.188
Abstract
OBJECTIVE
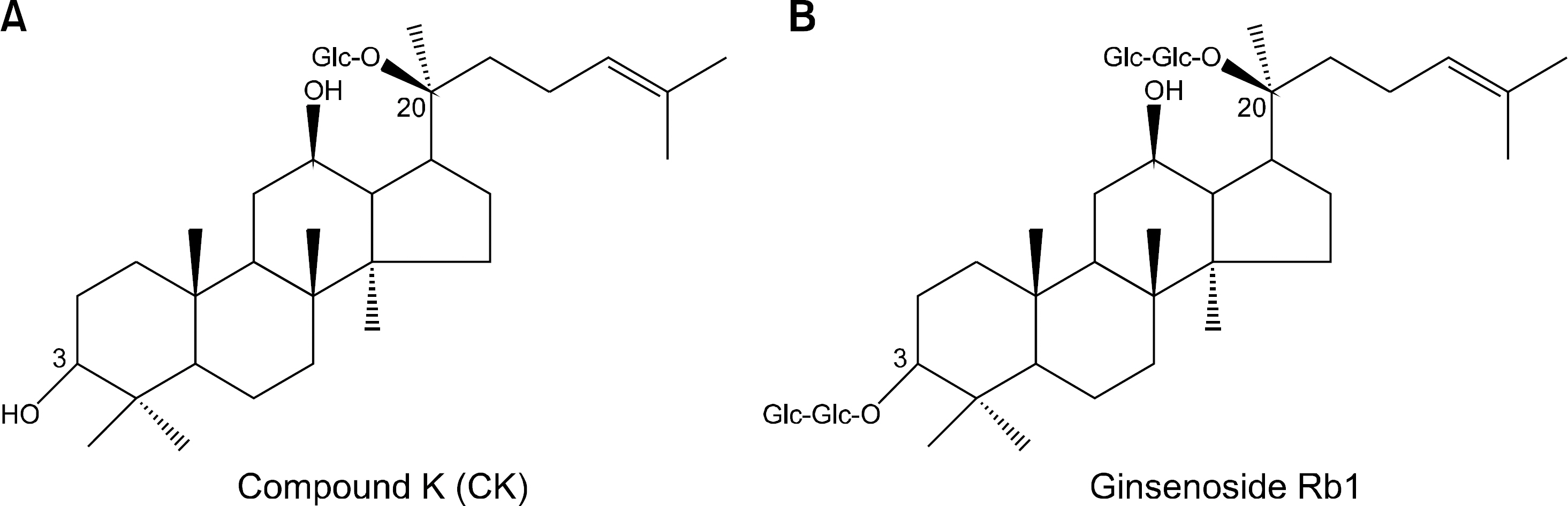
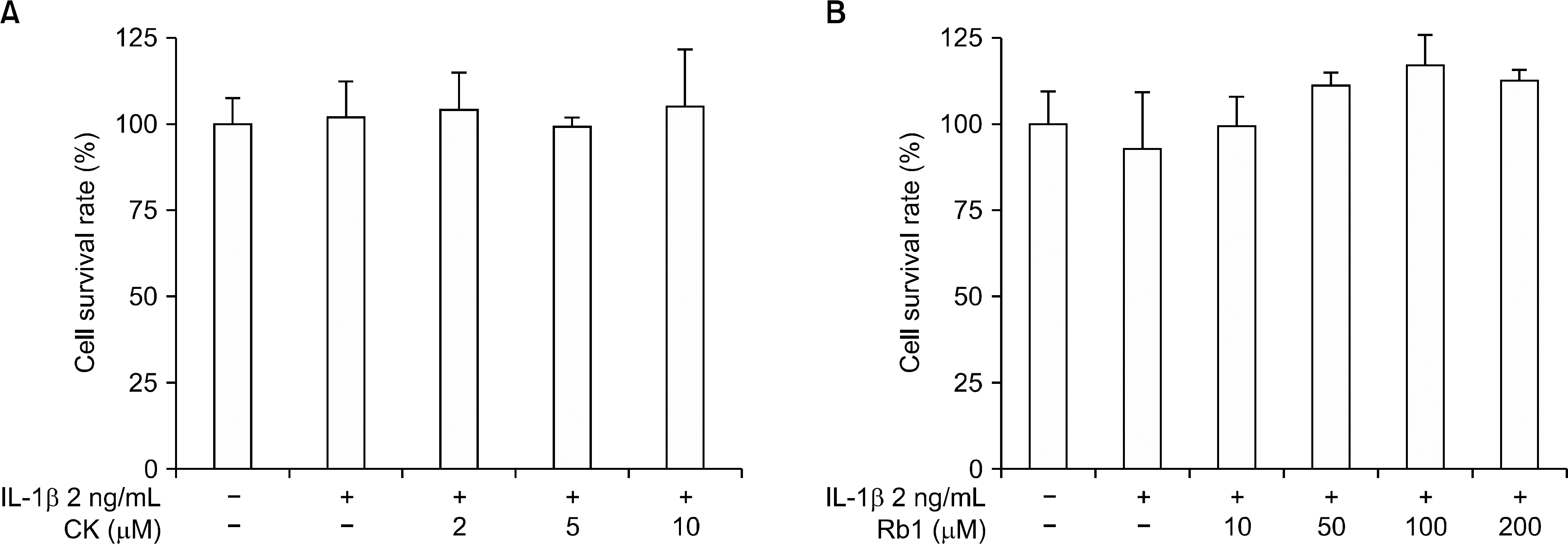
This study examined the anti-inflammatory and chondroprotective effects of compound K (CK), a ginsenoside metabolite, on chondrocytes from osteoarthritis (OA) patients following stimulation with interleukin (IL)-1β.
METHODS
Articular cartilage samples were obtained from six OA patients undergoing total knee replacement surgery. Nitric oxide (NO) production was measured by the Griess reaction. Subsequently, the mRNA and protein levels of matrix metalloproteinases (MMPs), inducible NO synthase (iNOS), and mitogen-activated protein kinases (MAPKs) were examined by a reverse transcription-polymerase chain reaction and western blot analysis. Cartilage degradation was assessed using a glycosaminoglycan (GAG) assay.
RESULTS
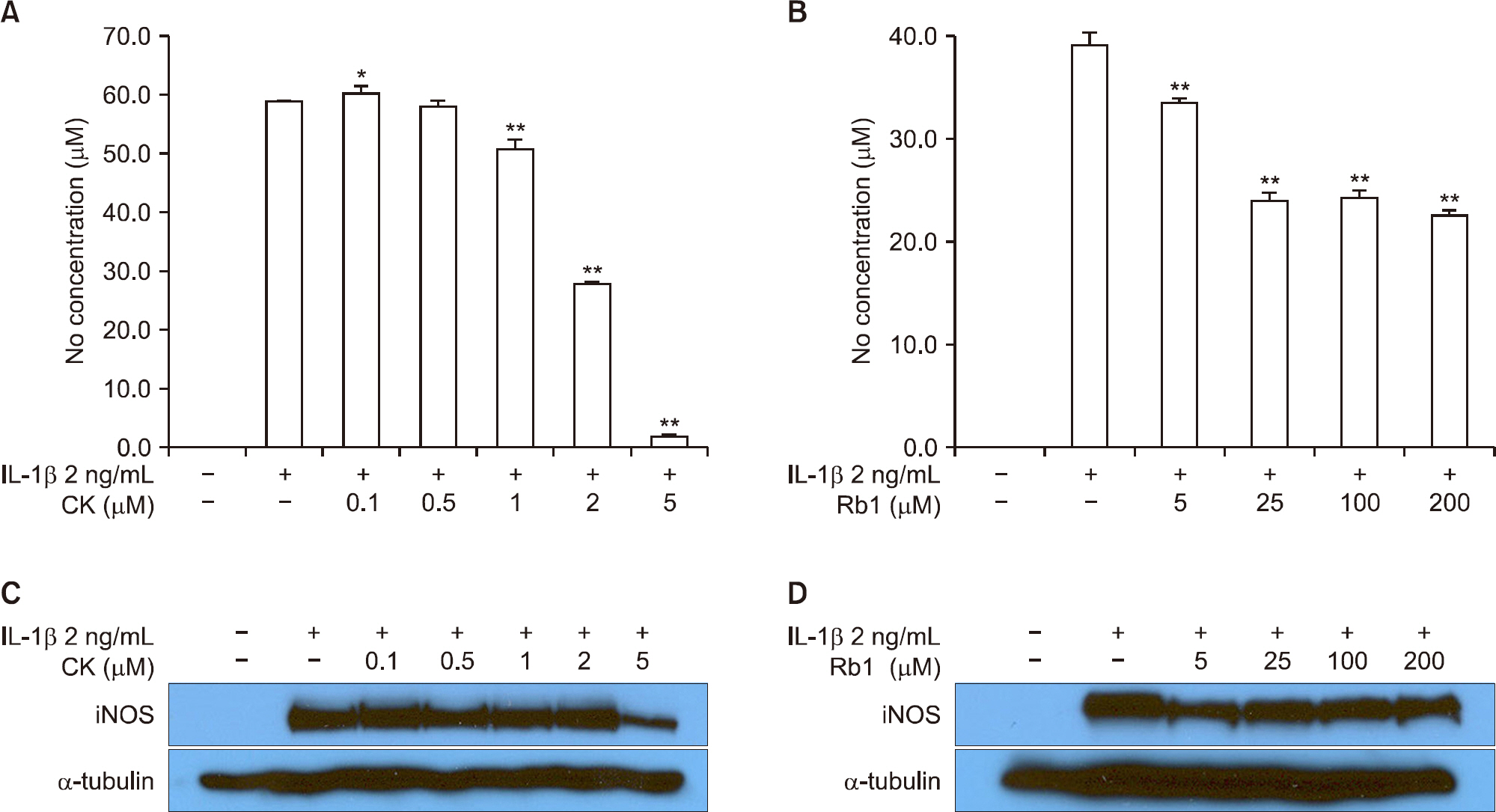
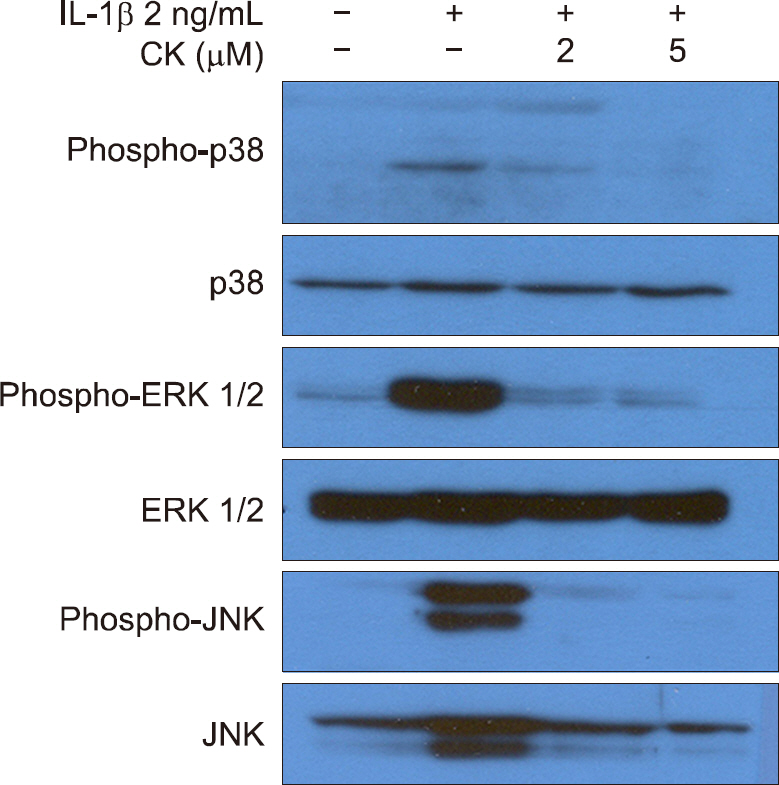
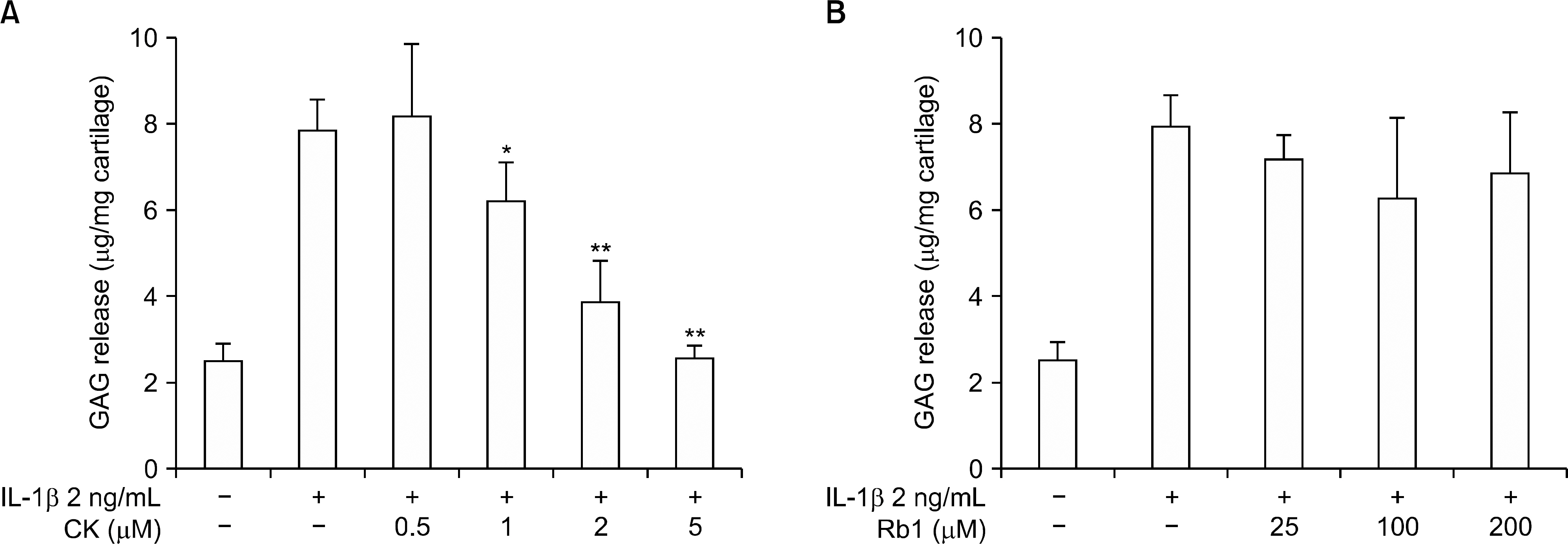
CK inhibited IL-1β-induced NO production and iNOS expression in a dose-dependent manner. In addition, it inhibited the IL-1 β-stimulated release of MMP-1, -3, and -13 and tissue inhibitor of matrix metalloproteinase-1 from OA patient chondrocytes. In addition, CK effectively suppressed the IL-1β-induced phosphorylation of p38, extracellular signal-regulated kinase-1/2, and c-Jun N-terminal kinase MAPKs. Moreover, the IL-1β-mediated release of GAG was inhibited by CK in a dose-dependent manner.
CONCLUSION
CK inhibited the IL-1β-induced expression of inflammatory mediators and MMPs by, at least in part, inhibiting MAPK activation, and has potential as a therapeutic agent for the treatment of OA.
MeSH Terms
-
Arthroplasty, Replacement, Knee
Blotting, Western
Cartilage
Cartilage, Articular
Chondrocytes*
Ginsenosides
Humans
Interleukin-1
Interleukins
JNK Mitogen-Activated Protein Kinases
Matrix Metalloproteinase 1
Matrix Metalloproteinases*
Mitogen-Activated Protein Kinases
Nitric Oxide
Nitric Oxide Synthase
Osteoarthritis
Panax
Phosphorylation
Protein Kinases*
RNA, Messenger
Ginsenosides
Interleukin-1
Interleukins
JNK Mitogen-Activated Protein Kinases
Matrix Metalloproteinase 1
Matrix Metalloproteinases
Mitogen-Activated Protein Kinases
Nitric Oxide
Nitric Oxide Synthase
Protein Kinases
RNA, Messenger
Figure
Cited by 1 articles
-
Causal Association between Bone Mineral Density and Osteoarthritis: A Mendelian Randomization Study
Gwan Gyu Song, Young Ho Lee
J Rheum Dis. 2019;26(2):104-110. doi: 10.4078/jrd.2019.26.2.104.
Reference
-
1. Centers for Disease Control and Prevention (CDC). Prevalence of doctor-diagnosed arthritis and arthritis-at-tributable activity limitation – United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2010; 59:1261–5.2. Liu-Bryan R. Inflammation and intracellular metabolism: new targets in OA. Osteoarthritis Cartilage. 2015; 23:1835–42.
Article3. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012; 64:1697–707.
Article4. Melchiorri C, Meliconi R, Frizziero L, Silvestri T, Pulsatelli L, Mazzetti I, et al. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1998; 41:2165–74.
Article5. Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993; 52:870–5.
Article6. Martel-Pelletier J, McCollum R, DiBattista J, Faure MP, Chin JA, Fournier S, et al. The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes. Identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum. 1992; 35:530–40.
Article7. Bian Q, Wang YJ, Liu SF, Li YP. Osteoarthritis: genetic factors, animal models, mechanisms, and therapies. Front Biosci (Elite Ed). 2012; 4:74–100.
Article8. Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006; 11:529–43.
Article9. Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-, MMP-13) genes in arthritis: in-tegration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002; 4:157–64.
Article10. Berenbaum F. Signaling transduction: target in osteoarthritis. Curr Opin Rheumatol. 2004; 16:616–22.
Article11. Sondergaard BC, Schultz N, Madsen SH, Bay-Jensen AC, Kassem M, Karsdal MA. MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation–divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthritis Cartilage. 2010; 18:279–88.12. Hwang HS, Park IY, Kim DW, Choi SY, Jung YO, Kim HA. PEP-1-FK506BP12 inhibits matrix metalloproteinase expression in human articular chondrocytes and in a mouse carrageenan-induced arthritis model. BMB Rep. 2015; 48:407–12.
Article13. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012; 64:465–74.
Article14. Farkouh ME, Greenberg JD, Jeger RV, Ramanathan K, Verheugt FW, Chesebro JH, et al. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007; 66:764–70.
Article15. Yuan HD, Kim JT, Kim SH, Chung SH. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res. 2012; 36:27–39.
Article16. Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004; 95:153–7.
Article17. Choi YS, Kang EH, Lee EY, Gong HS, Kang HS, Shin K, et al. Joint-protective effects of compound K, a major ginsenoside metabolite, in rheumatoid arthritis: in vitro evidence. Rheumatol Int. 2013; 33:1981–90.
Article18. Lee YJ, Song KY, Lee EY, Kang HS, Song YW. Compound K, a metabolite of ginsenosides, attenuates collagen-induced arthritis in mice. J Rheum Dis. 2015; 22:154–66.
Article19. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986; 29:1039–49.20. Cheng AW, Stabler TV, Bolognesi M, Kraus VB. Selenomethionine inhibits IL-1β inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2) expression in primary human chondrocytes. Osteoarthritis Cartilage. 2011; 19:118–25.
Article21. Huang CY, Hung LF, Liang CC, Ho LJ. COX-2 and iNOS are critical in advanced glycation end product-activated chondrocytes in vitro. Eur J Clin Invest. 2009; 39:417–28.22. Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005; 52:128–35.23. Chen WP, Wang YL, Tang JL, Hu PF, Bao JP, Wu LD. Morin inhibits interleukin-1β-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Int Immunopharmacol. 2012; 12:447–52.
Article24. Sasaki K, Hattori T, Fujisawa T, Takahashi K, Inoue H, Takigawa M. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J Biochem. 1998; 123:431–9.
Article25. Lewis EJ, Bishop J, Bottomley KM, Bradshaw D, Brewster M, Broadhurst MJ, et al. Ro 32-3555, an orally active collagenase inhibitor, prevents cartilage breakdown in vitro and in vivo. Br J Pharmacol. 1997; 121:540–6.26. Bonassar LJ, Frank EH, Murray JC, Paguio CG, Moore VL, Lark MW, et al. Changes in cartilage composition and physical properties due to stromelysin degradation. Arthritis Rheum. 1995; 38:173–83.
Article27. Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014; 39:25–32.
Article28. Liacini A, Sylvester J, Li WQ, Huang W, Dehnade F, Ahmad M, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-, and NF-kappaB transcription factors in articular chondrocytes. Exp Cell Res. 2003; 288:208–17.29. Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford). 2008; 47:409–14.
Article30. Akhtar N, Haqqi TM. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res Ther. 2011; 13:R93.
Article31. Rhule A, Navarro S, Smith JR, Shepherd DM. Panax noto-ginseng attenuates LPS-induced proinflammatory mediators in RAW264.7 cells. J Ethnopharmacol. 2006; 106:121–8.
Article32. Joh EH, Lee IA, Jung IH, Kim DH. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation–the key step of inflammation. Biochem Pharmacol. 2011; 82:278–86.
Article33. Jia P, Chen G, Li R, Rong X, Zhou G, Zhong Y. Ginsenoside Rb1 reduces nitric oxide production via inhibition of nuclear factor-κ B activation in interleukin-1β-stimulated SW1353 chondrosarcoma cells. Trop J Pharm Res. 2014; 13:1071.34. Jia L, Zhao Y, Liang XJ. Current evaluation of the millennium phytomedicine-ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications ema-nated from traditional Chinese medicine. Curr Med Chem. 2009; 16:2924–42.35. Park JS, Shin JA, Jung JS, Hyun JW, Van Le TK, Kim DH, et al. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J Pharmacol Exp Ther. 2012; 341:59–67.
Article36. Lee JY, Shin JW, Chun KS, Park KK, Chung WY, Bang YJ, et al. Antitumor promotional effects of a novel intestinal bacterial metabolite (IH-901) derived from the proto-panaxadiol-type ginsenosides in mouse skin. Carcinogenesis. 2005; 26:359–67.
Article37. Bezerra MM, Brain SD, Greenacre S, Jerônimo SM, de Melo LB, Keeble J, et al. Reactive nitrogen species scavenging, rather than nitric oxide inhibition, protects from articular cartilage damage in rat zymosan-induced arthritis. Br J Pharmacol. 2004; 141:172–82.
Article38. Chevalier X, Ravaud P, Maheu E, Baron G, Rialland A, Vergnaud P, et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis. 2015; 74:1697–705.
Article39. Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010; 16:678–86.40. Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010; 16:687–93.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SOCS1 suppresses IL-1β-induced C/EBPβ expression via transcriptional regulation in human chondrocytes
- Effects of gangliosides from deer bone extract on the gene expressions of matrix metalloproteinases and collagen type II in interleukin-1β-induced osteoarthritic chondrocytes
- A New Neolignan Derivative, Balanophonin Isolated from Firmiana simplex Delays the Progress of Neuronal Cell Death by Inhibiting Microglial Activation
- Dexamethasone Inhibits Interleukin-1beta-Induced Matrix Metalloproteinase-9 Expression in Cochlear Cells
- Baicalin suppresses lipopolysaccharide-induced matrix metalloproteinase expression: action via the mitogenactivated protein kinase and nuclear factor κB-related protein signaling pathway