Nutr Res Pract.
2016 Dec;10(6):569-574. 10.4162/nrp.2016.10.6.569.
Effects of gangliosides from deer bone extract on the gene expressions of matrix metalloproteinases and collagen type II in interleukin-1β-induced osteoarthritic chondrocytes
- Affiliations
-
- 1Department of Public Health Sciences, Korea University, Seoul 02841, Korea.
- 2Lotte Confectionery Co. Ltd, Seoul 07207, Korea.
- 3Nong Shim Co. LTD, Seoul 07057, Korea.
- 4Department of Home Economic Education, Jeonju University, Cheonjam-ro, Wansan-gu, Jeonju 55069, Korea. jjjj@jj.ac.kr
- KMID: 2359612
- DOI: http://doi.org/10.4162/nrp.2016.10.6.569
Abstract
- BACKGROUND/OBJECTIVES
We investigated the anti-osteoarthritic effects of deer bone extract on the gene expressions of matrix metalloproteinases (MMPs) and collagen type II (COL2) in interleukin-1β-induced osteoarthritis (OA) chondrocytes.
MATERIALS/METHODS
Primary rabbit chondrocytes were treated as follows: CON (PBS treatment), NC (IL-1β treatment), PC (IL-1β + 100 µg/mL glucosamine sulphate/chondroitin sulphate mixture), and DB (IL-1β + 100 µg/mL deer bone extract).
RESULTS
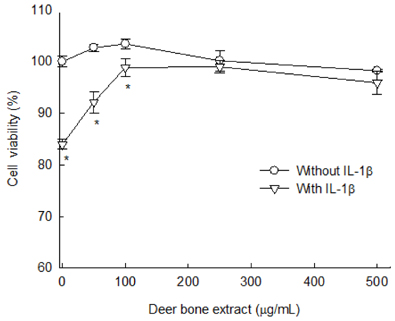
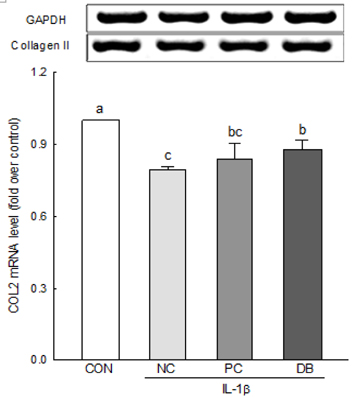
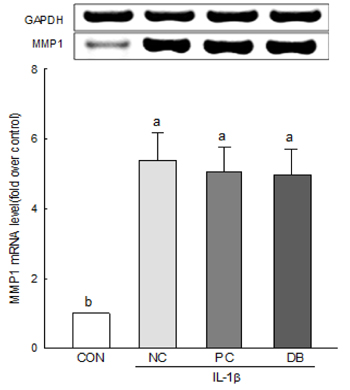
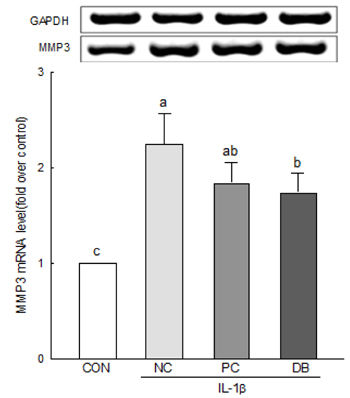
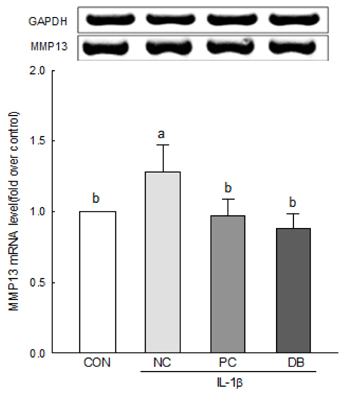
The results of the cell viability assay indicated that deer bone extract at doses ranging from 100 to 500 µg/mL inhibits cell death in chondrocytes induced by IL-1β. Deer bone extract was able to significantly recover the mRNA expression of COL2 that was down-regulated by IL-1β (NC: 0.79 vs. DB: 0.87, P < 0.05) and significantly decrease the mRNA expression of MMP-3 (NC: 2.24 vs. DB: 1.75) and -13 (NC: 1.28 vs. DB: 0.89) in OA chondrocytes (P < 0.05).
CONCLUSIONS
We concluded that deer bone extract induces accumulation of COL2 through the down-regulation of MMPs in IL-1β-induced OA chondrocytes. Our results suggest that deer bone extract, which contains various components related to OA, including chondroitin sulphate, may possess anti-osteoarthritic properties and be of value in inhibiting the pathogenesis of OA.
MeSH Terms
Figure
Reference
-
1. Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2005; 13:988–997.
Article2. Sanchez C, Deberg MA, Bellahcène A, Castronovo V, Msika P, Delcour JP, Crielaard JM, Henrotin YE. Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone. Arthritis Rheum. 2008; 58:442–455.
Article3. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013; 21:1145–1153.
Article4. Rashad S, Revell P, Hemingway A, Low F, Rainsford K, Walker F. Effect of non-steroidal anti-inflammatory drugs on the course of osteoarthritis. Lancet. 1989; 2:519–522.
Article5. Kean WF, Kean R, Buchanan WW. Osteoarthritis: symptoms, signs and source of pain. Inflammopharmacology. 2004; 12:3–31.
Article6. Leeb BF, Schweitzer H, Montag K, Smolen JS. A metaanalysis of chondroitin sulfate in the treatment of osteoarthritis. J Rheumatol. 2000; 27:205–211.7. Pravin SK, Durgacharab AB, Dinesh MS. Deer antlers-traditional use and future perspectives. Indian J Tradit Knowl. 2010; 9:245–251.8. Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967; 18:267–273.
Article9. Sunwoo HH, Nakano T, Sim JS. Isolation and characterization of proteoglycans from growing antlers of wapiti (Cervus elaphus). Comp Biochem Physiol B Biochem Mol Biol. 1998; 121:437–442.
Article10. Pham PH, Duffy TL, Dmytrash AL, Lien VW, Thomson AB, Clandinin MT. Estimate of dietary ganglioside intake in a group of healthy Edmontonians based on selected foods. J Food Compost Anal. 2011; 24:1032–1037.
Article11. Hara S, Yamaguchi M, Takemori Y, Furuhata K, Ogura H, Nakamura M. Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal Biochem. 1989; 179:162–166.
Article12. Salcedo J, Lacomba R, Alegria A, Barbera R, Matencio E, Lagarda MJ. Comparison of spectrophotometric and HPLC methods for determining sialic acid in infant formulas. Food Chem. 2011; 127:1905–1910.
Article13. Barbosa I, Garcia S, Barbier-Chassefière V, Caruelle JP, Martelly I, Papy-García D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003; 13:647–653.
Article14. Cheung HS, Harvey W, Benya PD, Nimni ME. New collagen markers of 'derepression' synthesized by rabbit articular chondrocytes in culture. Biochem Biophys Res Commun. 1976; 68:1371–1378.
Article15. Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988; 48:589–601.16. Marlovits S, Hombauer M, Truppe M, Vècsei V, Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Joint Surg Br. 2004; 86:286–295.
Article17. Lee SM, Moon J, Do HJ, Chung JH, Lee KH, Cha YJ, Shin MJ. Onion peel extract increases hepatic low-density lipoprotein receptor and ATP-binding cassette transporter A1 messenger RNA expressions in Sprague-Dawley rats fed a high-fat diet. Nutr Res. 2012; 32:210–217.
Article18. Sui Z, Zhang L, Huo Y, Zhang Y. Bioactive components of velvet antlers and their pharmacological properties. J Pharm Biomed Anal. 2014; 87:229–240.
Article19. Zhang L, Mu X, Fu J, Zhou Z. In vitro cytotoxicity assay with selected chemicals using human cells to predict target-organ toxicity of liver and kidney. Toxicol In Vitro. 2007; 21:734–740.
Article20. Yasuhara R, Miyamoto Y, Akaike T, Akuta T, Nakamura M, Takami M, Morimura N, Yasu K, Kamijo R. Interleukin-1β induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J. 2005; 389:315–323.
Article21. Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, Poole AR. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994; 93:1722–1732.
Article22. Chandrasekhar S, Harvey AK, Higginbotham JD, Horton WE. Interleukin-1-induced suppression of type II collagen gene transcription involves DNA regulatory elements. Exp Cell Res. 1990; 191:105–114.
Article23. Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001; 44:585–594.
Article24. Wolfe GC, MacNaul KL, Buechel FF, McDonnell J, Hoerrner LA, Lark MW, Moore VL, Hutchinson NI. Differential in vivo expression of collagenase messenger RNA in synovium and cartilage. Quantitative comparison with stromelysin messenger RNA levels in human rheumatoid arthritis and osteoarthritis patients and in two animal models of acute inflammatory arthritis. Arthritis Rheum. 1993; 36:1540–1547.25. Ma B, van Blitterswijk CA, Karperien M. A Wnt/beta-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum. 2012; 64:2589–2600.
Article26. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011; 3:a005058.
Article27. Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005; 52:128–135.
Article28. Nerucci F, Fioravanti A, Cicero MR, Collodel G, Marcolongo R. Effects of chondroitin sulfate and interleukin-1β on human chondrocyte cultures exposed to pressurization: a biochemical and morphological study. Osteoarthritis Cartilage. 2000; 8:279–287.
Article29. Wang L, Wang J, Almqvist KF, Veys EM, Verbruggen G. Influence of polysulphated polysaccharides and hydrocortisone on the extracellular matrix metabolism of human articular chondrocytes in vitro. Clin Exp Rheumatol. 2002; 20:669–676.30. Schwartz NB. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J Biol Chem. 1977; 252:6316–6321.
Article31. Schwartz NB, Dorfman A. Stimulation of chondroitin sulfate proteoglycan production by chondrocytes in monolayer. Connect Tissue Res. 1975; 3:115–122.
Article32. Uebelhart D, Thonar EJ, Zhang J, Williams JM. Protective effect of exogenous chondroitin 4,6-sulfate in the acute degradation of articular cartilage in the rabbit. Osteoarthritis Cartilage. 1998; 6 Suppl A. 6–13.
Article33. Chan PS, Caron JP, Orth MW. Effect of glucosamine and chondroitin sulfate on regulation of gene expression of proteolytic enzymes and their inhibitors in interleukin-1-challenged bovine articular cartilage explants. Am J Vet Res. 2005; 66:1870–1876.
Article34. Lippiello L, Woodward J, Karpman R, Hammad TA. In vivo chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clin Orthop Relat Res. 2000; 229–240.
Article35. Hakomori SI. Structure and function of glycosphingolipids and sphingolipids: Recollections and future trends. Biochim Biophys Acta. 2008; 1780:325–346.
Article36. Seito N, Yamashita T, Tsukuda Y, Matsui Y, Urita A, Onodera T, Mizutani T, Haga H, Fujitani N, Shinohara Y, Minami A, Iwasaki N. Interruption of glycosphingolipid synthesis enhances osteoarthritis development in mice. Arthritis Rheum. 2012; 64:2579–2588.
Article37. David MJ, Hellio MP, Portoukalian J, Richard M, Caton J, Vignon E. Gangliosides from normal and osteoarthritic joints. J Rheumatol Suppl. 1995; 43:133–135.38. David MJ, Portoukalian J, Rebbaa A, Vignon E, Carret JP, Richard M. Characterization of gangliosides from normal and osteoarthritic human articular cartilage. Arthritis Rheum. 1993; 36:938–942.
Article39. Tsukuda Y, Iwasaki N, Seito N, Kanayama M, Fujitani N, Shinohara Y, Kasahara Y, Onodera T, Suzuki K, Asano T, Minami A, Yamashita T. Ganglioside GM3 has an essential role in the pathogenesis and progression of rheumatoid arthritis. PLoS One. 2012; 7:e40136.
Article40. Sasazawa F, Onodera T, Yamashita T, Seito N, Tsukuda Y, Fujitani N, Shinohara Y, Iwasaki N. Depletion of gangliosides enhances cartilage degradation in mice. Osteoarthritis Cartilage. 2014; 22:313–322.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of the Expression of Matrix Metalloproteinases in Articular Chondrocytes by Resveratrol through Affecting Nuclear Factor-Kappa B Signaling Pathway
- Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo
- The Effects of Dehydroepiandrosterone-pyruvate on Human Osteoarthritic Chondrocytes
- Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat
- Effect of Hijikia fusiforme extracts on degenerative osteoarthritis in vitro and in vivo models