Korean J Physiol Pharmacol.
2018 Jul;22(4):379-389. 10.4196/kjpp.2018.22.4.379.
Adenine attenuates lipopolysaccharide-induced inflammatory reactions
- Affiliations
-
- 1Department of Medical Science, College of Medicine, Chungnam National University, Daejeon 35015, Korea. parksk@cnu.ac.kr
- 2Department of Biochemistry, College of Medicine, Chungnam National University, Daejeon 35015, Korea.
- 3Department of Oriental Medicine, Daejeon University, Daejeon 34520, Korea.
- KMID: 2414262
- DOI: http://doi.org/10.4196/kjpp.2018.22.4.379
Abstract
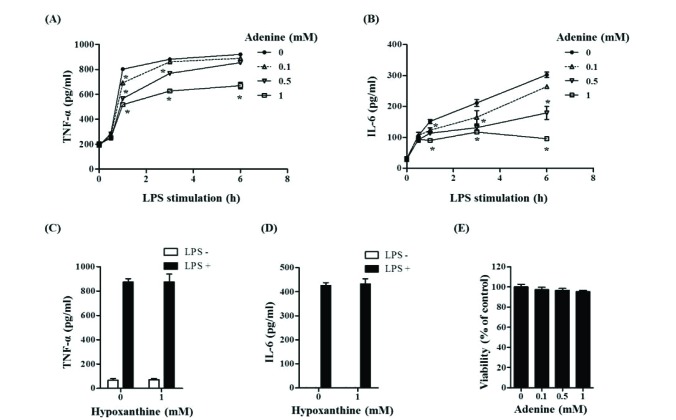
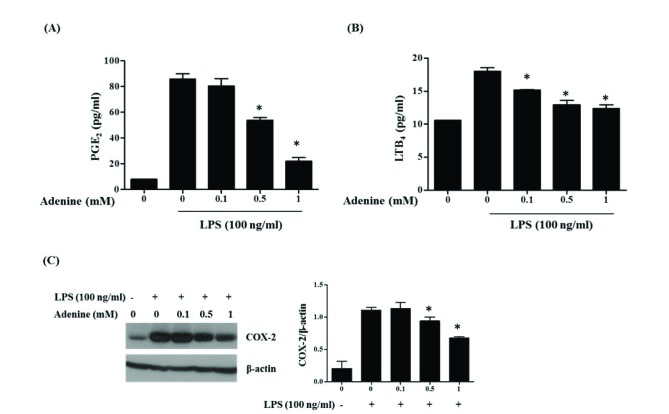
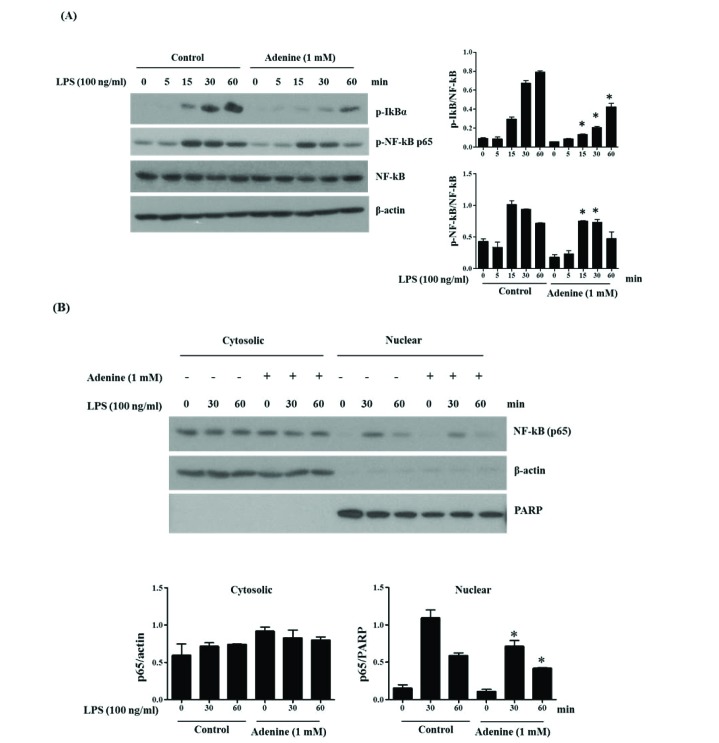
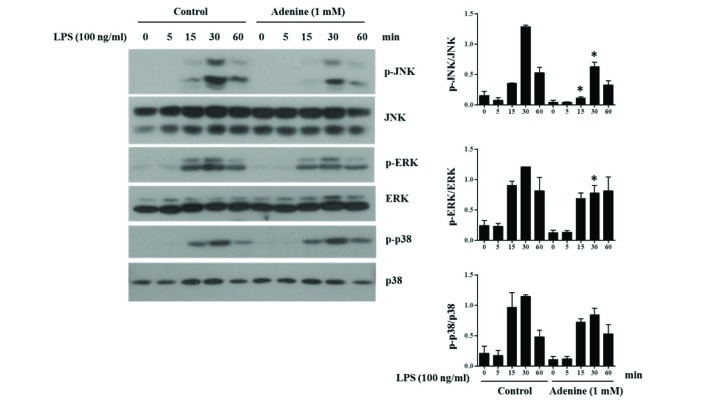
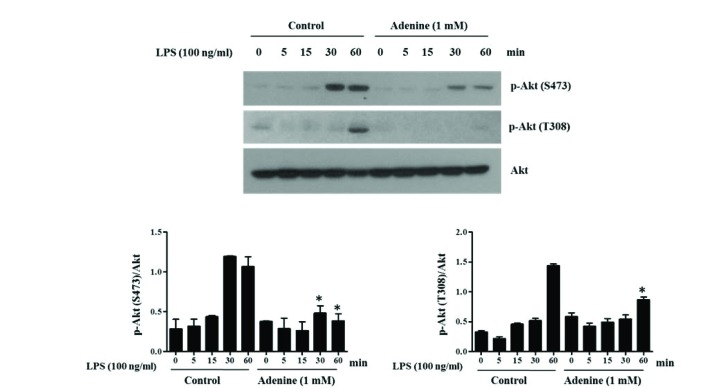
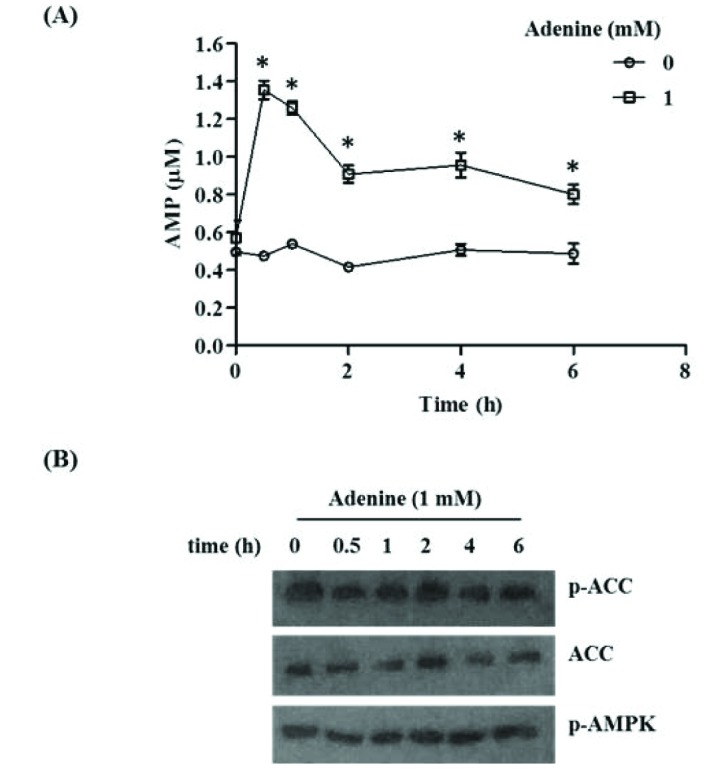
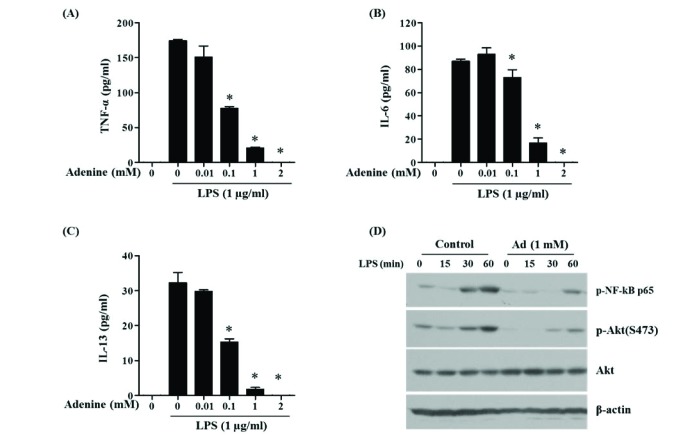
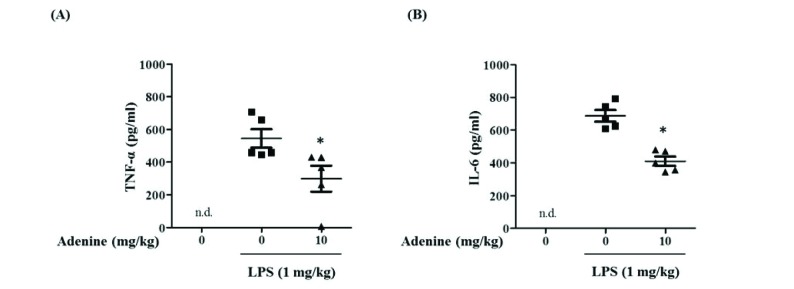
- A nucleobase adenine is a fundamental component of nucleic acids and adenine nucleotides. Various biological roles of adenine have been discovered. It is not produced from degradation of adenine nucleotides in mammals but produced mainly during polyamine synthesis by dividing cells. Anti-inflammatory roles of adenine have been supported in IgE-mediated allergic reactions, immunological functions of lymphocytes and dextran sodium sulfate-induced colitis. However adenine effects on Toll-like receptor 4 (TLR4)-mediated inflammation by lipopolysaccharide (LPS), a cell wall component of Gram negative bacteria, is not examined. Here we investigated anti-inflammatory roles of adenine in LPS-stimulated immune cells, including a macrophage cell line RAW264.7 and bone marrow derived mast cells (BMMCs) and peritoneal cells in mice. In RAW264.7 cells stimulated with LPS, adenine inhibited production of pro-inflammatory cytokines TNF-α and IL-6 and inflammatory lipid mediators, prostaglandin E₂ and leukotriene B₄. Adenine impeded signaling pathways eliciting production of these inflammatory mediators. It suppressed IκB phosphorylation, nuclear translocation of nuclear factor κB (NF-κB), phosphorylation of Akt and mitogen activated protein kinases (MAPKs) JNK and ERK. Although adenine raised cellular AMP which could activate AMP-dependent protein kinase (AMPK), the enzyme activity was not enhanced. In BMMCs, adenine inhibited the LPS-induced production of TNF-α, IL-6 and IL-13 and also hindered phosphorylation of NF-κB and Akt. In peritoneal cavity, adenine suppressed the LPS-induced production of TNF-α and IL-6 by peritoneal cells in mice. These results show that adenine attenuates the LPS-induced inflammatory reactions.
MeSH Terms
-
Adenine Nucleotides
Adenine*
Animals
Bone Marrow
Cell Line
Cell Wall
Colitis
Cytokines
Dextrans
Gram-Negative Bacteria
Hypersensitivity
Inflammation
Interleukin-13
Interleukin-6
Lymphocytes
Macrophages
Mammals
Mast Cells
Mice
Mitogen-Activated Protein Kinases
Nucleic Acids
Peritoneal Cavity
Phosphorylation
Protein Kinases
Sodium
Toll-Like Receptor 4
Adenine
Adenine Nucleotides
Cytokines
Dextrans
Interleukin-13
Interleukin-6
Mitogen-Activated Protein Kinases
Nucleic Acids
Protein Kinases
Sodium
Toll-Like Receptor 4
Figure
Reference
-
1. Kishi T, Kittaka E, Hyodo S, Kashiwa H, Karakawa T, Suzawa T, Sakura N, Sakano T, Usui T. Inhibition by adenine of in vitro immunological functions of normal and adenine phosphoribosyltransferase-deficient human lymphocytes. Immunopharmacology. 1985; 10:157–162. PMID: 3833854.
Article2. Hershfield MS, Snyder FF, Seegmiller JE. Adenine and adenosine are toxic to human lymphoblast mutants defective in purine salvage enzymes. Science. 1977; 197:1284–1287. PMID: 197600.
Article3. Watanabe S, Yoshimi Y, Ikekita M. Neuroprotective effect of adenine on purkinje cell survival in rat cerebellar primary cultures. J Neurosci Res. 2003; 74:754–759. PMID: 14635226.
Article4. Avila MA, García-Trevijano ER, Lu SC, Corrales FJ, Mato JM. Methylthioadenosine. Int J Biochem Cell Biol. 2004; 36:2125–2130. PMID: 15313459.
Article5. Kamatani N, Carson DA. Dependence of adenine production upon polyamine synthesis in cultured human lymphoblasts. Biochim Biophys Acta. 1981; 675:344–350. PMID: 6791702.
Article6. Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013; 18:556–566. PMID: 24093679.
Article7. Bender E, Buist A, Jurzak M, Langlois X, Baggerman G, Verhasselt P, Ercken M, Guo HQ, Wintmolders C, Van den Wyngaert I, Van Oers I, Schoofs L, Luyten W. Characterization of an orphan G protein-coupled receptor localized in the dorsal root ganglia reveals adenine as a signaling molecule. Proc Natl Acad Sci U S A. 2002; 99:8573–8578. PMID: 12084918.
Article8. von Kügelgen I, Schiedel AC, Hoffmann K, Alsdorf BB, Abdelrahman A, Müller CE. Cloning and functional expression of a novel Gi protein-coupled receptor for adenine from mouse brain. Mol Pharmacol. 2008; 73:469–477. PMID: 17975009.9. Wengert M, Adão-Novaes J, Assaife-Lopes N, Leão-Ferreira LR, Caruso-Neves C. Adenine-induced inhibition of Na+-ATPase activity: Evidence for involvement of the Gi protein-coupled receptor in the cAMP signaling pathway. Arch Biochem Biophys. 2007; 467:261–267. PMID: 17892855.10. Simon ER, Chapman RG, Finch CA. Adenine in red cell preservation. J Clin Invest. 1962; 41:351–359. PMID: 14039291.
Article11. Tokuda S, Fukuda T, Kobayashi Y, Tanaka M, Matsui T. Effect of the uncharged imidazolium moiety in adenine on endothelium-independent relaxation in the contracted thoracic aorta of Sprague-Dawley rats. Biosci Biotechnol Biochem. 2012; 76:828–830. PMID: 22484931.
Article12. Snyder FF, Hershfield MS, Seegmiller JE. Cytotoxic and metabolic effects of adenosine and adenine on human lymphoblasts. Cancer Res. 1978; 38:2357–2362. PMID: 667833.13. Ito K, Uchino H. Control of pyrimidine biosynthesis in human lymphocytes. Inhibitory effect of guanine and guanosine on induction of enzymes for pyrimidine biosynthesis de novo in phytohemagglutinin-stimulated lymphocytes. J Biol Chem. 1976; 251:1427–1430. PMID: 1254576.
Article14. Silwal P, Shin K, Choi S, Kang SW, Park JB, Lee HJ, Koo SJ, Chung KH, Namgung U, Lim K, Heo JY, Park JI, Park SK. Adenine suppresses IgE-mediated mast cell activation. Mol Immunol. 2015; 65:242–249. PMID: 25700347.
Article15. Fukuda T, Majumder K, Zhang H, Turner PV, Matsui T, Mine Y. Adenine inhibits TNF-α signaling in intestinal epithelial cells and reduces mucosal inflammation in a dextran sodium sulfate-induced colitis mouse model. J Agric Food Chem. 2016; 64:4227–4234. PMID: 27166765.
Article16. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/ HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998; 282:2085–2088. PMID: 9851930.17. Savva A, Roger T. Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol. 2013; 4:387. PMID: 24302927.
Article18. Paterson RL, Webster NR. Sepsis and the systemic inflammatory response syndrome. J R Coll Surg Edinb. 2000; 45:178–182. PMID: 10881485.19. Herzum I, Renz H. Inflammatory markers in SIRS, sepsis and septic shock. Curr Med Chem. 2008; 15:581–587. PMID: 18336272.20. De Nardo D. Toll-like receptors: activation, signalling and transcriptional modulation. Cytokine. 2015; 74:181–189. PMID: 25846205.
Article21. Zhang YL, Dong C. MAP kinases in immune responses. Cell Mol Immunol. 2005; 2:20–27. PMID: 16212907.22. Schorey JS, Cooper AM. Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003; 5:133–142. PMID: 12614457.
Article23. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007; 7:353–364. PMID: 17457343.24. Madrid LV, Mayo MW, Reuther JY, Baldwin AS Jr. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001; 276:18934–18940. PMID: 11259436.25. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010; 327:656–661. PMID: 20133564.
Article26. Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, Ma GF, Konttinen YT. Regulation of macrophage activation. Cell Mol Life Sci. 2003; 60:2334–2346. PMID: 14625680.
Article27. Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol. 2012; 3:185. PMID: 22783258.
Article28. Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004; 4:787–799. PMID: 15459670.
Article29. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–384. PMID: 20404851.
Article30. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001; 294:1871–1875. PMID: 11729303.
Article31. Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NF-kappaB complex. Cell. 1998; 95:749–758. PMID: 9865693.32. Cheng YF, Young GH, Lin JT, Jang HH, Chen CC, Nong JY, Chen PK, Kuo CY, Kao SH, Liang YJ, Chen HM. Activation of AMP-activated protein kinase by adenine alleviates TNF-alpha-induced inflammation in human umbilical vein endothelial cells. PLoS One. 2015; 10:e0142283. PMID: 26544976.
Article33. Young GH, Lin JT, Cheng YF, Huang CF, Chao CY, Nong JY, Chen PK, Chen HM. Identification of adenine modulating AMPK activation in NIH/3T3 cells by proteomic approach. J Proteomics. 2015; 120:204–214. PMID: 25797921.
Article34. Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999; 179(Suppl 2):S294–S304. PMID: 10081499.
Article35. Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol. 1998; 275:R269–R277. PMID: 9688988.
Article36. Dahdah A, Gautier G, Attout T, Fiore F, Lebourdais E, Msallam R, Daëron M, Monteiro RC, Benhamou M, Charles N, Davoust J, Blank U, Malissen B, Launay P. Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis. J Clin Invest. 2014; 124:4577–4589. PMID: 25180604.
Article37. Ajuebor MN, Das AM, Virág L, Flower RJ, Szabó C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999; 162:1685–1691. PMID: 9973430.38. Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001; 107:135–142. PMID: 11160126.39. Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999; 274:27339–27342. PMID: 10488062.40. Hochdörfer T, Kuhny M, Zorn CN, Hendriks RW, Vanhaesebroeck B, Bohnacker T, Krystal G, Huber M. Activation of the PI3K pathway increases TLR-induced TNF-α and IL-6 but reduces IL-1β production in mast cells. Cell Signal. 2011; 23:866–875. PMID: 21262348.
Article41. Kuo CL, Ho FM, Chang MY, Prakash E, Lin WW. Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 gene expression by 5-aminoimidazole-4-carboxamide riboside is independent of AMP-activated protein kinase. J Cell Biochem. 2008; 103:931–940. PMID: 17615555.
Article42. Jhun BS, Jin Q, Oh YT, Kim SS, Kong Y, Cho YH, Ha J, Baik HH, Kang I. 5-Aminoimidazole-4-carboxamide riboside suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of phosphatidylinositol 3-kinase/Akt activation in RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 2004; 318:372–380. PMID: 15120611.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Adenine on Clozapine-induced Neutropenia in Patients with Schizophrenia: A Preliminary Study
- Efficacy of Adenine in the Treatment of Leukopenia and Neutropenia Associated with an Overdose of Antipsychotics or Discontinuation of Lithium Carbonate Administration: Three Case Studies
- Effects of an Herbal Medicinal Product Composed of Three Herbal Materials on Lipopolysaccharide-induced Depression-like Behaviors in Mice
- Anti-inflammatory effects of rutin in lipopolysaccharide-stimulated canine macrophage cells
- Erratum to "Suppression of Lipopolysaccharide-induced Inflammatory and Oxidative Response by 5-Aminolevulinic Acid in RAW 264.7 Macrophages and Zebrafish Larvae" [Biomol Ther 29(6), 685-696 (2021)]