J Periodontal Implant Sci.
2018 Feb;48(1):34-46. 10.5051/jpis.2018.48.1.34.
Effects of 1,25-dihydroxyvitamin D₃ on the differentiation of MC3T3-E1 osteoblast-like cells
- Affiliations
-
- 1Department of Periodontology, Chosun University School of Dentistry, Gwangju, Korea. bobkim@chosun.ac.kr
- 2Department of Stomatology, Affiliated Hospital of Yanbian University, Yanji, China.
- 3Department of Oral Physiology, Chosun University School of Dentistry, Gwangju, Korea.
- KMID: 2405408
- DOI: http://doi.org/10.5051/jpis.2018.48.1.34
Abstract
- PURPOSE
The purpose of this study was to evaluate the effects of 1,25-dihydroxyvitamin D₃ on the proliferation, differentiation, and matrix mineralization of MC3T3-E1 osteoblast-like cells in vitro.
METHODS
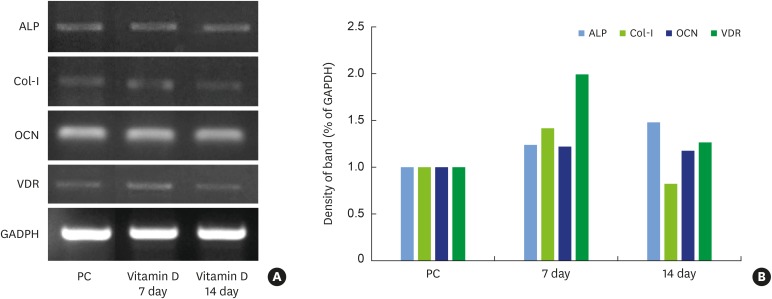
MC3T3-E1 osteoblastic cells and 1,25-dihydroxyvitamin D₃ were prepared. Cytotoxic effects and osteogenic differentiation were evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, alkaline phosphatase (ALP) activity assay, ALP staining, alizarin red S staining, and reverse transcription-polymerase chain reaction (RT-PCR) for osteogenic differentiation markers such as ALP, collagen type I (Col-I), osteocalcin (OCN), vitamin D receptor (VDR), and glyceraldehyde 3-phosphate dehydrogenase.
RESULTS
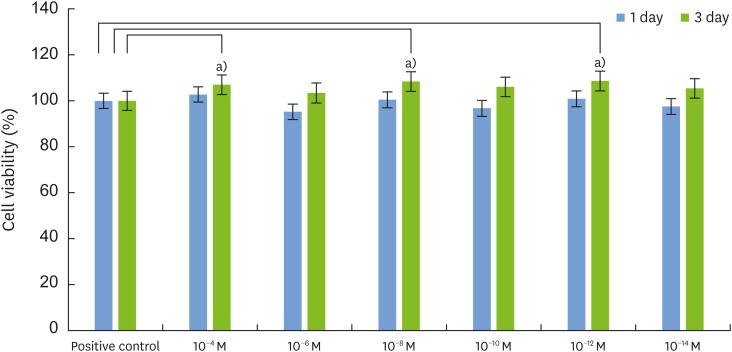
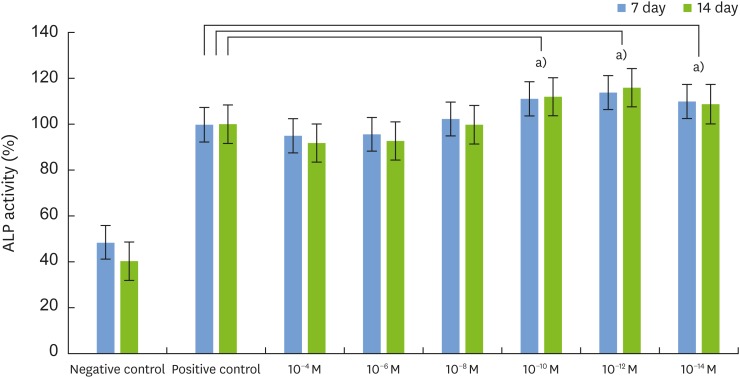
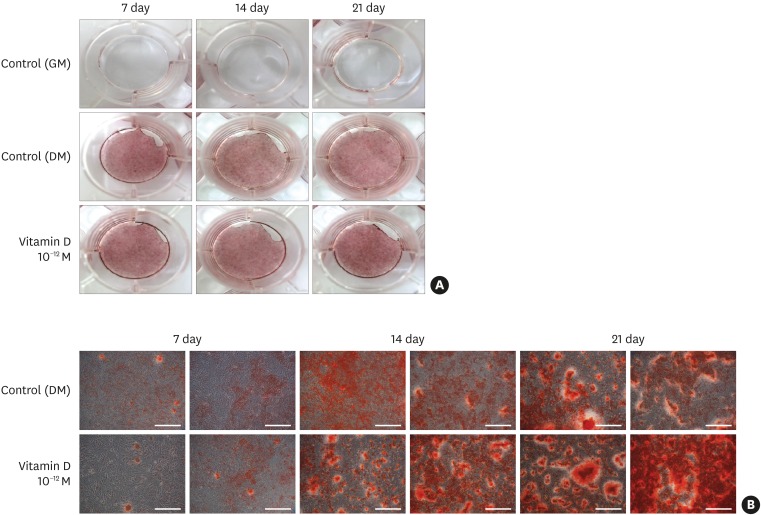
The MTT assay showed that 1,25-dihydroxyvitamin D₃ did not inhibit cell growth and that the rate of cell proliferation was higher than in the positive control group at all concentrations. ALP activity was also higher than in the positive control group at low concentrations of 1,25-dihydroxyvitamin D₃ (10−10, 10−12, and 10−14 M). RT-PCR showed that the gene expression levels of ALP, Col-I, OCN, and vitamin D receptor (VDR) were higher at a low concentration of 1,25-dihydroxyvitamin D₃ (10−12 M). Alizarin red S staining after treatment with 1,25-dihydroxyvitamin D₃ (10−12 M) showed no significant differences in the overall degree of calcification. In contrast to the positive control group, formation of bone nodules was induced in the early stages of cell differentiation.
CONCLUSIONS
We suggest that 1,25-dihydroxyvitamin D₃ positively affects cell differentiation and matrix mineralization. Therefore, it may function as a stimulating factor in osteoblastic bone formation and can be used as an additive in bone regeneration treatment.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Antigens, Differentiation
Bone Regeneration
Calcitriol
Cell Differentiation
Cell Proliferation
Collagen Type I
Gene Expression
Glyceraldehyde 3-Phosphate
In Vitro Techniques
Miners
Osteoblasts
Osteocalcin
Osteogenesis
Oxidoreductases
Receptors, Calcitriol
Alkaline Phosphatase
Antigens, Differentiation
Calcitriol
Collagen Type I
Glyceraldehyde 3-Phosphate
Osteocalcin
Oxidoreductases
Receptors, Calcitriol
Figure
Reference
-
1. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003; 23:313–323. PMID: 12956475.2. Hynes K, Menicanin D, Gronthos S, Bartold PM. Clinical utility of stem cells for periodontal regeneration. Periodontol 2000. 2012; 59:203–227. PMID: 22507067.
Article3. Rosenberg E, Rose LF. Biologic and clinical considerations for autografts and allografts in periodontal regeneration therapy. Dent Clin North Am. 1998; 42:467–490. PMID: 9700450.4. Fiorellini JP, Kim DM, Nakajima Y, Weber HP. Osseointegration of titanium implants following guided bone regeneration using expanded polytetrafluoroethylene membrane and various bone fillers. Int J Periodontics Restorative Dent. 2007; 27:287–294. PMID: 17694952.5. Nakashima M. Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev. 2005; 16:369–376. PMID: 15878301.
Article6. Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003; 21:1025–1032. PMID: 12949568.
Article7. Srisuwan T, Tilkorn DJ, Wilson JL, Morrison WA, Messer HM, Thompson EW, et al. Molecular aspects of tissue engineering in the dental field. Periodontol 2000. 2006; 41:88–108. PMID: 16686928.
Article8. Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr Pharm Biotechnol. 2002; 3:129–139. PMID: 12022256.
Article9. Minghetti PP, Norman AW. 1,25-dihydroxy-vitamin D3 receptors: gene regulation and genetic circuitry. FASEB J. 1988; 2:3043–3053. PMID: 2847948.10. Henry HL, Norman AW. Studies on the mechanism of action of calciferol VII. Localization of 1,25-dihydroxy-vitamin D3 in chick parathyroid glands. Biochem Biophys Res Commun. 1975; 62:781–788. PMID: 164191.
Article11. Minghetti PP, Norman AW. 1,25(OH)2-vitamin D3 receptors: gene regulation and genetic circuitry. FASEB J. 1988; 2:3043–3053. PMID: 2847948.12. Norman AW. Vitamin D: the calcium homeostatic steroid hormone. New York (NY): Academic Press;1979.13. Norman AW, Roth J, Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982; 3:331–366. PMID: 6295752.14. Haneji T, Kurihara N, Ikeda K, Kumegawa M. 1α,25-Dihydroxyvitamin D3 and analogues of vitamin D3 induce alkaline phosphatase activity in osteoblastic cells derived from newborn mouse calvaria. J Biochem. 1983; 94:1127–1132. PMID: 6317662.
Article15. Matsumoto T, Igarashi C, Takeuchi Y, Harada S, Kikuchi T, Yamato H, et al. Stimulation by 1, 25-dihydroxyvitamin D3 of in vitro mineralization induced by osteoblast-like MC3T3-E1 cells. Bone. 1991; 12:27–32. PMID: 2054233.16. Seibert J, Nyman S. Localized ridge augmentation in dogs: a pilot study using membranes and hydroxyapatite. J Periodontol. 1990; 61:157–165. PMID: 2156985.
Article17. Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, Bourguignon M, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000; 18:959–963. PMID: 10973216.
Article18. Choi JY, Lee BH, Song KB, Park RW, Kim IS, Sohn KY, et al. Expression patterns of bone related proteins during osteoblastic differentiation in MC3T3E1 cells. J Cell Biochem. 1996; 61:609–618. PMID: 8806085.19. Lian JB, Stein GS, Canalis E, Robey PG, Boskey AL. Bone formation: osteoblast lineage cells, growth factors, matrix proteins and the mineralization process. In : Favus M, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins;1999. p. 14–19.20. Zheng MZ, Lee SY, Yu SJ, Kim BO. Effect of Cornus officinalis extract on the differentiation of MC3T3-E1 osteoblast-like cells. Tissue Eng Regen Med. 2015; 12:113–121.
Article21. Spelsberg TC, Subramaniam M, Riggs BL, Khosla S. The actions and interactions of sex steroids and growth factors/cytokines on the skeleton. Mol Endocrinol. 1999; 13:819–828. PMID: 10379881.
Article22. Fraser JD, Otawara Y, Price PA. 1,25-Dihydroxyvitamin D3 stimulates the synthesis of matrix gamma-carboxyglutamic acid protein by osteosarcoma cells. Mutually exclusive expression of vitamin K-dependent bone proteins by clonal osteoblastic cell lines. J Biol Chem. 1988; 263:911–916. PMID: 3257212.
Article23. Manolagas SC, Burton DW, Deftos LJ. 1,25-Dihydroxyvitamin D3 stimulates the alkaline phosphatase activity of osteoblast-like cells. J Biol Chem. 1981; 256:7115–7117. PMID: 6941964.
Article24. Price PA, Baukol SA. 1,25-Dihydroxyvitamin D3 increases synthesis of the vitamin K-dependent bone protein by osteosarcoma cells. J Biol Chem. 1980; 255:11660–11663. PMID: 6969260.
Article25. Rowe DW, Kream BE. Regulation of collagen synthesis in fetal rat calvaria by 1,25-dihydroxyvitamin D3. J Biol Chem. 1982; 257:8009–8015. PMID: 6896329.
Article26. Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013; 92:77–98. PMID: 22782502.
Article27. Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: integrated actions of a well-defined transcription factor. Steroids. 2013; 78:127–136. PMID: 23178257.
Article28. Franceschi RT, Ge C, Xiao G, Roca H, Jiang D. Transcriptional regulation of osteoblasts. Cells Tissues Organs. 2009; 189:144–152. PMID: 18728356.
Article29. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008; 88:582S–586S. PMID: 18689406.
Article30. Sigurdsson TJ, Nygaard L, Tatakis DN, Fu E, Turek TJ, Jin L, et al. Periodontal repair in dogs: evaluation of rhBMP-2 carriers. Int J Periodontics Restorative Dent. 1996; 16:524–537. PMID: 9242091.31. Kurihara N, Ishizuka S, Kiyoki M, Haketa Y, Ikeda K, Kumegawa M. Effects of 1,25-dihydroxyvitamin D3 on osteoblastic MC3T3-E1 cells. Endocrinology. 1986; 118:940–947. PMID: 3004901.32. Lee SY, Kim SG, Lee SA, Park MG, Park SY, Oh JS, et al. Influence of Carthamus tinctorius seed extract on proliferation and differentiation of MC3T3-E1 osteoblast cells. Oral Biol Res. 2013; 37:73–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of 1,25-Dihydroxyvitamin D3 on Expression of IGF-I Gene and Cellular Proliferation in MC3T3-E1 Cells
- Effects of Ascorbic Acid on Osteoblast Differentiation in MC3T3-E1 Cells
- Zinc modulation of osterix in MC3T3-E1 cells
- The effects of vanadium oxide & sodium orthovanadate on murin osteoblast-like (MC3T3-E1) cells
- Cellular zinc deficiency inhibits the mineralized nodule formation and downregulates bone-specific gene expression in osteoblastic MC3T3-E1 cells