Immune Netw.
2018 Feb;18(1):e8. 10.4110/in.2018.18.e8.
Regulation of Osteoclast Differentiation by Cytokine Networks
- Affiliations
-
- 1Department of Microbiology and Molecular Biology, Chungnam National University, Daejeon 34134, Korea. jrrho@cnu.ac.kr
- KMID: 2404891
- DOI: http://doi.org/10.4110/in.2018.18.e8
Abstract
- Cytokines play a pivotal role in maintaining bone homeostasis. Osteoclasts (OCs), the sole bone resorbing cells, are regulated by numerous cytokines. Macrophage colony-stimulating factor and receptor activator of NF-κB ligand play a central role in OC differentiation, which is also termed osteoclastogenesis. Osteoclastogenic cytokines, including tumor necrosis factor-α, IL-1, IL-6, IL-7, IL-8, IL-11, IL-15, IL-17, IL-23, and IL-34, promote OC differentiation, whereas anti-osteoclastogenic cytokines, including interferon (IFN)-α, IFN-β, IFN-γ, IL-3, IL-4, IL-10, IL-12, IL-27, and IL-33, downregulate OC differentiation. Therefore, dynamic regulation of osteoclastogenic and anti-osteoclastogenic cytokines is important in maintaining the balance between bone-resorbing OCs and bone-forming osteoblasts (OBs), which eventually affects bone integrity. This review outlines the osteoclastogenic and anti-osteoclastogenic properties of cytokines with regard to osteoimmunology, and summarizes our current understanding of the roles these cytokines play in osteoclastogenesis.
MeSH Terms
-
Cytokines
Homeostasis
Interferons
Interleukin-1
Interleukin-10
Interleukin-11
Interleukin-12
Interleukin-15
Interleukin-17
Interleukin-23
Interleukin-27
Interleukin-3
Interleukin-33
Interleukin-4
Interleukin-6
Interleukin-7
Interleukin-8
Macrophage Colony-Stimulating Factor
Necrosis
Osteoblasts
Osteoclasts*
RANK Ligand
Cytokines
Interferons
Interleukin-1
Interleukin-10
Interleukin-11
Interleukin-12
Interleukin-15
Interleukin-17
Interleukin-23
Interleukin-3
Interleukin-33
Interleukin-4
Interleukin-6
Interleukin-7
Interleukin-8
Macrophage Colony-Stimulating Factor
RANK Ligand
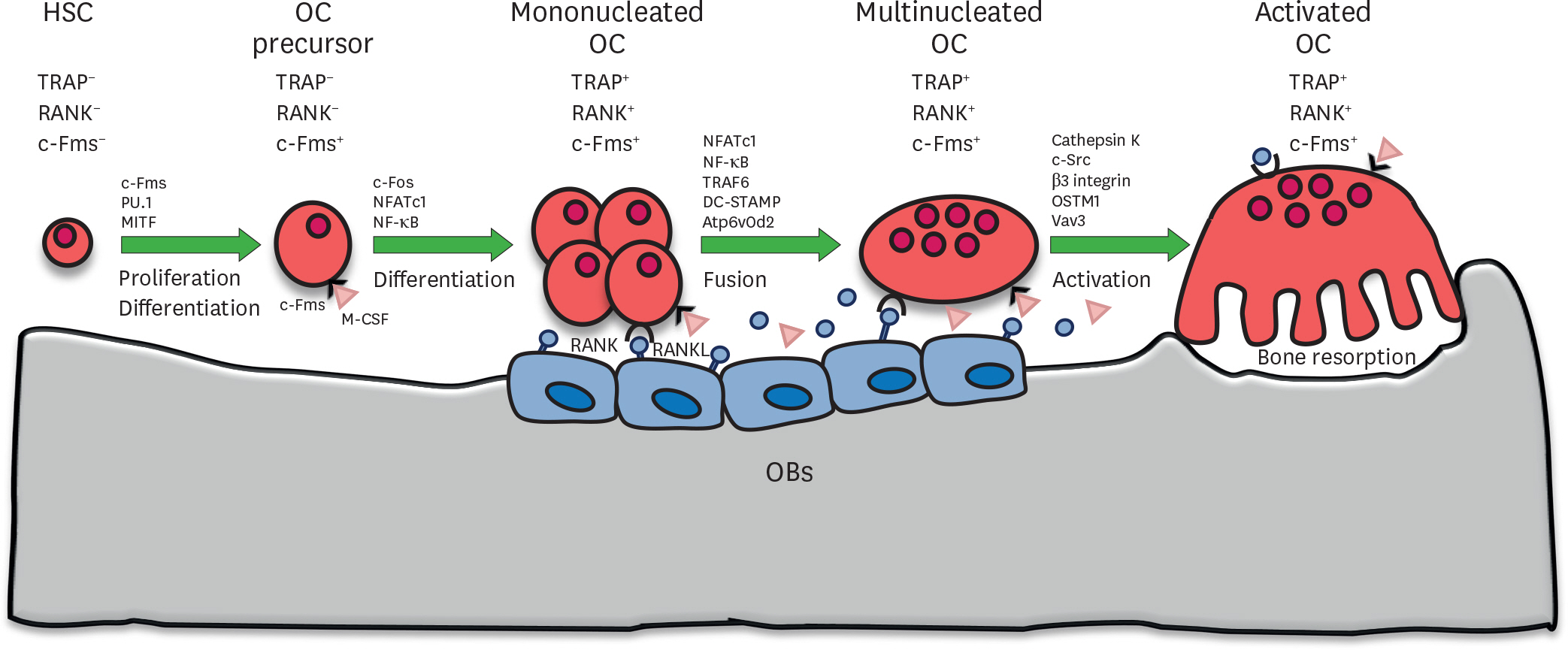
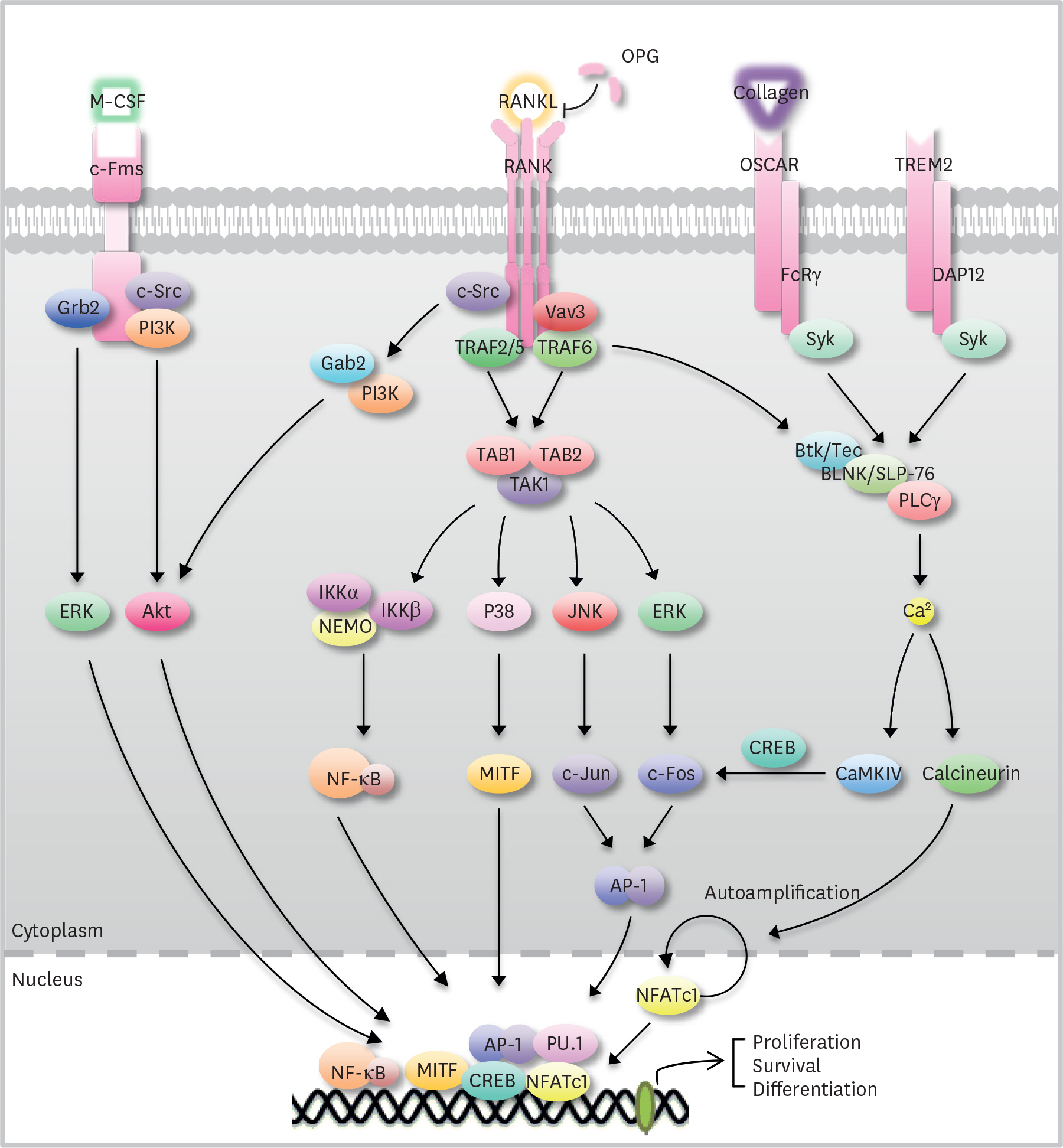
Figure
Reference
-
References
1. Rho J, Takami M, Choi Y. Osteoimmunology: interactions of the immune and skeletal systems. Mol Cells. 2004; 17:1–9.2. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007; 7:292–304.
Article3. Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006; 24:33–63.
Article4. Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997; 186:2075–2080.
Article5. Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998; 12:1260–1268.6. Amarasekara DS, Yu J, Rho J. Bone loss triggered by the cytokine network in inflammatory autoimmune diseases. J Immunol Res. 2015; 2015:832127.
Article7. Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990; 345:442–444.
Article8. Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990; 87:4828–4832.
Article9. Takahashi N, Udagawa N, Akatsu T, Tanaka H, Shionome M, Suda T. Role of colony-stimulating factors in osteoclast development. J Bone Miner Res. 1991; 6:977–985.
Article10. Yu J, Yun H, Shin B, Kim Y, Park ES, Choi S, Yu J, Amarasekara DS, Kim S, Inoue J, et al. Interaction of tumor necrosis factor receptor-associated factor 6 (TRAF6) and Vav3 in the receptor activator of nuclear factor κ B (RANK) signaling complex enhances osteoclastogenesis. J Biol Chem. 2016; 291:20643–20660.
Article11. Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002; 195:201–209.
Article12. Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK, et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006; 12:1403–1409.
Article13. Rho J, Altmann CR, Socci ND, Merkov L, Kim N, So H, Lee O, Takami M, Brivanlou AH, Choi Y. Gene expression profiling of osteoclast differentiation by combined suppression subtractive hybridization (SSH) and cDNA microarray analysis. DNA Cell Biol. 2002; 21:541–549.
Article14. Shin B, Yu J, Park ES, Choi S, Yu J, Hwang JM, Yun H, Chung YH, Hong KS, Choi JS, et al. Secretion of a truncated osteopetrosis-associated transmembrane protein 1 (OSTM1) mutant inhibits osteoclastogenesis through down-regulation of the B lymphocyte-induced maturation protein 1 (BLIMP1)-nuclear factor of activated T cells c1 (NFATc1) axis. J Biol Chem. 2014; 289:35868–35881.
Article15. Barrow AD, Raynal N, Andersen TL, Slatter DA, Bihan D, Pugh N, Cella M, Kim T, Rho J, Negishi-Koga T, et al. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J Clin Invest. 2011; 121:3505–3516.
Article16. Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004; 428:758–763.
Article17. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014; 5:511.
Article18. Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000; 275:4858–4864.19. Komine M, Kukita A, Kukita T, Ogata Y, Hotokebuchi T, Kohashi O. Tumor necrosis factor-alpha cooperates with receptor activator of nuclear factor kappaB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone. 2001; 28:474–483.20. Kanazawa K, Azuma Y, Nakano H, Kudo A. TRAF5 functions in both RANKL- and TNFalpha-induced osteoclastogenesis. J Bone Miner Res. 2003; 18:443–450.21. Kanazawa K, Kudo A. TRAF2 is essential for TNF-alpha-induced osteoclastogenesis. J Bone Miner Res. 2005; 20:840–847.22. Park ES, Choi S, Shin B, Yu J, Yu J, Hwang JM, Yun H, Chung YH, Choi JS, Choi Y, et al. Tumor necrosis factor (TNF) receptor-associated factor (TRAF)-interacting protein (TRIP) negatively regulates the TRAF2 ubiquitin-dependent pathway by suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction. J Biol Chem. 2015; 290:9660–9673.
Article23. Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. Tumor necrosis factor-α (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem. 2001; 276:563–568.
Article24. Yao Z, Lei W, Duan R, Li Y, Luo L, Boyce BF. RANKL cytokine enhances TNF-induced osteoclastogenesis independently of TNF receptor associated factor (TRAF) 6 by degrading TRAF3 in osteoclast precursors. J Biol Chem. 2017; 292:10169–10179.
Article25. Kitaura H, Kimura K, Ishida M, Kohara H, Yoshimatsu M, Takano-Yamamoto T. Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin Dev Immunol. 2013; 2013:181849.26. Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest. 2005; 115:3418–3427.
Article27. Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000; 96:1873–1878.
Article28. Hwang SJ, Choi B, Kang SS, Chang JH, Kim YG, Chung YH, Sohn DH, So MW, Lee CK, Robinson WH, et al. Interleukin-34 produced by human fibroblast-like synovial cells in rheumatoid arthritis supports osteoclastogenesis. Arthritis Res Ther. 2012; 14:R14.
Article29. Corrado A, Neve A, Maruotti N, Cantatore FP. Bone effects of biologic drugs in rheumatoid arthritis. Clin Dev Immunol. 2013; 2013:945945.
Article30. Ruscitti P, Cipriani P, Carubbi F, Liakouli V, Zazzeroni F, Di Benedetto P, Berardicurti O, Alesse E, Giacomelli R. The role of IL-1β in the bone loss during rheumatic diseases. Mediators Inflamm. 2015; 2015:782382.31. Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005; 115:282–290.
Article32. Jules J, Zhang PAshley JW, Wei S, Shi Z, Liu J, Michalek SM, Feng X. Molecular basis of requirement of receptor activator of nuclear factor κ B signaling for interleukin 1-mediated osteoclastogenesis. J Biol Chem. 2012; 287:15728–15738.
Article33. Kim JH, Jin HM, Kim K, Song I, Youn BU, Matsuo K, Kim N. The mechanism of osteoclast differentiation induced by IL-1. J Immunol. 2009; 183:1862–1870.
Article34. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012; 11:633–652.
Article35. Rose-John S. Interleukin-6 family cytokines. Cold Spring Harb Perspect Biol.DOI: doi: 10.1101/cshperspect.a028415.
Article36. Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem. 2008; 283:11535–11540.37. Axmann R, Böhm C, Krönke G, Zwerina J, Smolen J, Schett G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009; 60:2747–2756.38. Blanchard F, Duplomb L, Baud'huin M, Brounais B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009; 20:19–28.
Article39. Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003; 32:1–7.
Article40. Feng W, Liu H, Luo T, Liu D, Du J, Sun J, Wang W, Han X, Yang K, Guo J, et al. Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-κ B, ERK and JNK signaling pathways. Sci Rep. 2017; 7:41411.
Article41. Aguila HL, Mun SH, Kalinowski J, Adams DJ, Lorenzo JA, Lee SK. Osteoblast-specific overexpression of human interleukin-7 rescues the bone mass phenotype of interleukin-7-deficient female mice. J Bone Miner Res. 2012; 27:1030–1042.
Article42. Toraldo G, Roggia C, Qian WP, Pacifici R, Weitzmann MN. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc Natl Acad Sci U S A. 2003; 100:125–130.43. Yu J, Choi S, Kim H, Lee N, Yun H, Kim S, Jeong ST, Rho J. Generation of an osteoblast-based artificial niche that supports in vitro B lymphopoiesis. Exp Mol Med. 2017; 49:e400.44. Roato I, Brunetti G, Gorassini E, Grano M, Colucci S, Bonello L, Buffoni L, Manfredi R, Ruffini E, Ottaviani D, et al. IL-7 up-regulates TNF-α-dependent osteoclastogenesis in patients affected by solid tumor. PLoS One. 2006; 1:e124.
Article45. Kim JH, Sim JH, Lee S, Seol MA, Ye SK, Shin HM, Lee EB, Lee YJ, Choi YJ, Yoo WH, et al. Interleukin-7 induces osteoclast formation via STAT5, independent of receptor activator of NF-kappaB ligand. Front Immunol. 2017; 8:1376.
Article46. Lee SK, Kalinowski JF, Jastrzebski SL, Puddington L, Lorenzo JA. Interleukin-7 is a direct inhibitor of in vitro osteoclastogenesis. Endocrinology. 2003; 144:3524–3531.47. Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003; 33:28–37.
Article48. Kopesky P, Tiedemann K, Alkekhia D, Zechner C, Millard B, Schoeberl B, Komarova SV. Autocrine signaling is a key regulatory element during osteoclastogenesis. Biol Open. 2014; 3:767–776.
Article49. Liu Y, Krishnamurthy A, Hensvold AH, Joshua V, Sun M, Engstrom M, Wähämaa H, Malmström V, Jopling LA, Rethi B, et al. AB0078 Role of IL-8 and its receptor in anti-citrullinated protein antibody mediated osteoclastogenesis in RA. Ann Rheum Dis. 2016; 75:923.50. Girasole G, Passeri G, Jilka RL, Manolagas SC. Interleukin-11: a new cytokine critical for osteoclast development. J Clin Invest. 1994; 93:1516–1524.
Article51. McCoy EM, Hong H, Pruitt HC, Feng X. IL-11 produced by breast cancer cells augments osteoclastogenesis by sustaining the pool of osteoclast progenitor cells. BMC Cancer. 2013; 13:16.
Article52. Morgan H, Tumber A, Hill PA. Breast cancer cells induce osteoclast formation by stimulating host IL-11 production and downregulating granulocyte/macrophage colony-stimulating factor. Int J Cancer. 2004; 109:653–660.
Article53. Ogata Y, Kukita A, Kukita T, Komine M, Miyahara A, Miyazaki S, Kohashi O. A novel role of IL-15 in the development of osteoclasts: inability to replace its activity with IL-2. J Immunol. 1999; 162:2754–2760.54. Okabe I, Kikuchi T, Mogi M, Takeda H, Aino M, Kamiya Y, Fujimura T, Goto H, Okada K, Hasegawa Y, et al. IL-15 and RANKL play a synergistically important role in osteoclastogenesis J Cell Biochem. 2017; 118:739–747.
Article55. Lubberts E, van den Bersselaar L, Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJ, Kolls JK, Joosten LA, van den Berg WB. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-κ B ligand/osteoprotegerin balance. J Immunol. 2003; 170:2655–2662.56. Lubberts E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res Ther. 2005; 7:29–37.57. Hwang SY, Kim JY, Kim KW, Park MK, Moon Y, Kim WU, Kim HY. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther. 2004; 6:R120–R128.58. Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998; 160:3513–3521.59. Yago T, Nanke Y, Ichikawa N, Kobashigawa T, Mogi M, Kamatani N, Kotake S. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-alpha antibody: a novel mechanism of osteoclastogenesis by IL-17. J Cell Biochem. 2009; 108:947–955.60. Moon YM, Yoon BY, Her YM, Oh HJ, Lee JS, Kim KW, Lee SY, Woo YJ, Park KS, Park SH, et al. IL-32 and IL-17 interact and have the potential to aggravate osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther. 2012; 14:R246.
Article61. Kellner H. Targeting interleukin-17 in patients with active rheumatoid arthritis: rationale and clinical potential. Ther Adv Musculoskelet Dis. 2013; 5:141–152.
Article62. Balani D, Aeberli D, Hofstetter W, Seitz M. Interleukin-17A stimulates granulocyte-macrophage colony-stimulating factor release by murine osteoblasts in the presence of 1,25-dihydroxyvitamin D(3) and inhibits murine osteoclast development in vitro. Arthritis Rheum. 2013; 65:436–446.63. Chen L, Wei XQ, Evans B, Jiang W, Aeschlimann D. IL-23 promotes osteoclast formation by up-regulation of receptor activator of NF-kappaB (RANK) expression in myeloid precursor cells. Eur J Immunol. 2008; 38:2845–2854.64. Kang YK, Zhang MC. IL-23 promotes osteoclastogenesis in osteoblast-osteoclast co-culture system. Genet Mol Res. 2014; 13:4673–4679.
Article65. Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006; 116:1218–1222.
Article66. Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, Motoyoshi K, Kimura F, Okada S. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 2010; 17:1917–1927.
Article67. Chen Z, Buki K, Vääräniemi J, Gu G, Väänänen HK. The critical role of IL-34 in osteoclastogenesis. PLoS One. 2011; 6:e18689.
Article68. Cheng X, Wan QL, Li ZB. AG490 suppresses interleukin-34-mediated osteoclastogenesis in mice bone marrow macrophages. Cell Biol Int. 2017; 41:659–668.
Article69. Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Zhang L, Yuan ZR, Tan HS, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 2006; 16:126–133.
Article70. Lee MS, Kim HS, Yeon JT, Choi SW, Chun CH, Kwak HB, Oh J. GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. J Immunol. 2009; 183:3390–3399.
Article71. Ruef N, Dolder S, Aeberli D, Seitz M, Balani D, Hofstetter W. Granulocyte-macrophage colony-stimulating factor-dependent CD11c-positive cells differentiate into active osteoclasts. Bone. 2017; 97:267–277.
Article72. Atanga E, Dolder S, Dauwalder T, Wetterwald A, Hofstetter W. TNFα inhibits the development of osteoclasts through osteoblast-derived GM-CSF. Bone. 2011; 49:1090–1100.
Article73. Mackay CR. Chemokines: immunology's high impact factors. Nat Immunol. 2001; 2:95–101.
Article74. Lee SH, Kim TS, Choi Y, Lorenzo J. Osteoimmunology: cytokines and the skeletal system. BMB Rep. 2008; 41:495–510.
Article75. Kuan WP, Tam LS, Wong CK, Ko FW, Li T, Zhu T, Li EK. CXCL 9 and CXCL 10 as Sensitive markers of disease activity in patients with rheumatoid arthritis. J Rheumatol. 2010; 37:257–264.
Article76. Pathak JL, Bakker AD, Verschueren P, Lems WF, Luyten FP, Klein-Nulend J, Bravenboer N. CXCL8 and CCL20 enhance osteoclastogenesis via modulation of cytokine production by human primary osteoblasts. PLoS One. 2015; 10:e0131041.
Article77. Kwak HB, Ha H, Kim HN, Lee JH, Kim HS, Lee S, Kim HM, Kim JY, Kim HH, Song YW, et al. Reciprocal cross-talk between RANKL and interferon-gamma-inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis Rheum. 2008; 58:1332–1342.78. Ha J, Choi HS, Lee Y, Kwon HJ, Song YW, Kim HH. CXC chemokine ligand 2 induced by receptor activator of NF-kappa B ligand enhances osteoclastogenesis. J Immunol. 2010; 184:4717–4724.79. Koizumi K, Saitoh Y, Minami T, Takeno N, Tsuneyama K, Miyahara T, Nakayama T, Sakurai H, Takano Y, Nishimura M, et al. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J Immunol. 2009; 183:7825–7831.
Article80. Xuan W, Feng X, Qian C, Peng L, Shi Y, Xu L, Wang F, Tan W. Osteoclast differentiation gene expression profiling reveals chemokine CCL4 mediates RANKL-induced osteoclast migration and invasion via PI3K pathway. Cell Biochem Funct. 2017; 35:171–177.
Article81. Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003; 18:1404–1418.
Article82. Yu X, Huang Y, Collin-Osdoby P, Osdoby P. CCR1 chemokines promote the chemotactic recruitment, RANKL development, and motility of osteoclasts and are induced by inflammatory cytokines in osteoblasts. J Bone Miner Res. 2004; 19:2065–2077.
Article83. Xiong Q, Zhang L, Ge W, Tang P. The roles of interferons in osteoclasts and osteoclastogenesis. Joint Bone Spine. 2016; 83:276–281.
Article84. Avnet S, Cenni E, Perut F, Granchi D, Brandi ML, Giunti A, Baldini N. Interferon-alpha inhibits in vitro osteoclast differentiation and renal cell carcinoma-induced angiogenesis. Int J Oncol. 2007; 30:469–476.85. Lee Y, Hyung SW, Jung HJ, Kim HJ, Staerk J, Constantinescu SN, Chang EJ, Lee ZH, Lee SW, Kim HH. The ubiquitin-mediated degradation of Jak1 modulates osteoclastogenesis by limiting interferon-beta-induced inhibitory signaling. Blood. 2008; 111:885–893.86. Zheng H, Yu X, Collin-Osdoby P, Osdoby P. RANKL stimulates inducible nitric-oxide synthase expression and nitric oxide production in developing osteoclasts. An autocrine negative feedback mechanism triggered by RANKL-induced interferon-beta via NF-kappaB that restrains osteoclastogenesis and bone resorption. J Biol Chem. 2006; 281:15809–15820.87. Takayanagi H, Sato K, Takaoka A, Taniguchi T. Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev. 2005; 208:181–193.
Article88. Hayashida C, Ito J, Nakayachi M, Okayasu M, Ohyama Y, Hakeda Y, Sato T. Osteocytes produce interferon-β as a negative regulator of osteoclastogenesis. J Biol Chem. 2014; 289:11545–11555.
Article89. Ha H, Lee JH, Kim HN, Kwak HB, Kim HM, Lee SE, Rhee JH, Kim HH, Lee ZH. Stimulation by TLR5 modulates osteoclast differentiation through STAT1/IFN-beta. J Immunol. 2008; 180:1382–1389.90. Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007; 204:987–994.
Article91. Kopitar-Jerala N. The role of interferons in inflammation and inflammasome activation. Front Immunol. 2017; 8:873.
Article92. Makris S, Paulsen M, Johansson C. Type I interferons as regulators of lung inflammation. Front Immunol. 2017; 8:259.93. Thomas KE, Galligan CL, Newman RD, Fish EN, Vogel SN. Contribution of interferon-beta to the murine macrophage response to the toll-like receptor 4 agonist, lipopolysaccharide. J Biol Chem. 2006; 281:31119–31130.94. Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000; 408:600–605.
Article95. Kohara H, Kitaura H, Fujimura Y, Yoshimatsu M, Morita Y, Eguchi T, Masuyama R, Yoshida N. IFN-γ directly inhibits TNF-α-induced osteoclastogenesis in vitro and in vivo and induces apoptosis mediated by Fas/Fas ligand interactions. Immunol Lett. 2011; 137:53–61.96. Ji JD, Park-Min KH, Shen Z, Fajardo RJ, Goldring SR, McHugh KP, Ivashkiv LB. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-gamma in human osteoclast precursors. J Immunol. 2009; 183:7223–7233.97. Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4 (+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999; 67:2804–2809.98. Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007; 117:122–132.99. Kim JW, Lee MS, Lee CH, Kim HY, Chae SU, Kwak HB, Oh J. Effect of interferon-γ on the fusion of mononuclear osteoclasts into bone-resorbing osteoclasts. BMB Rep. 2012; 45:281–286.
Article100. Gupta N, Barhanpurkar AP, Tomar GB, Srivastava RK, Kour S, Pote ST, Mishra GC, Wani MR. IL-3 inhibits human osteoclastogenesis and bone resorption through downregulation of c-Fms and diverts the cells to dendritic cell lineage. J Immunol. 2010; 185:2261–2272.
Article101. Lee J, Seong S, Kim JH, Kim K, Kim I, Jeong BC, Nam KI, Kim KK, Hennighausen L, Kim N. STAT5 is a key transcription factor for IL-3-mediated inhibition of RANKL-induced osteoclastogenesis. Sci Rep. 2016; 6:30977.
Article102. Yogesha SD, Khapli SM, Wani MR. Interleukin-3 and granulocyte-macrophage colony-stimulating factor inhibits tumor necrosis factor (TNF)-α-induced osteoclast differentiation by down-regulation of expression of TNF receptors 1 and 2. J Biol Chem. 2005; 280:11759–11769.
Article103. Wei S, Wang MW, Teitelbaum SL, Ross FP. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-kappa B and mitogen-activated protein kinase signaling. J Biol Chem. 2002; 277:6622–6630.104. Cheng J, Liu J, Shi Z, Xu D, Luo S, Siegal GP, Feng X, Wei S. Interleukin-4 inhibits RANKL-induced NFATc1 expression via STAT6: a novel mechanism mediating its blockade of osteoclastogenesis. J Cell Biochem. 2011; 112:3385–3392.
Article105. te Velde AA, Huijbens RJ, Heije K, de Vries JE, Figdor CG. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990; 76:1392–1397.
Article106. Stein NC, Kreutzmann C, Zimmermann SP, Niebergall U, Hellmeyer L, Goettsch C, Schoppet M, Hofbauer LC. Interleukin-4 and interleukin-13 stimulate the osteoclast inhibitor osteoprotegerin by human endothelial cells through the STAT6 pathway. J Bone Miner Res. 2008; 23:750–758.
Article107. Onoe Y, Miyaura C, Kaminakayashiki T, Nagai Y, Noguchi K, Chen QR, Seo H, Ohta H, Nozawa S, Kudo I, et al. IL-13 and IL-4 inhibit bone resorption by suppressing cyclooxygenase-2-dependent prostaglandin synthesis in osteoblasts. J Immunol. 1996; 156:758–764.108. Evans KE, Fox SW. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007; 8:4.
Article109. Liu D, Yao S, Wise GE. Effect of interleukin-10 on gene expression of osteoclastogenic regulatory molecules in the rat dental follicle. Eur J Oral Sci. 2006; 114:42–49.
Article110. Houri-Haddad Y, Soskolne WA, Halabi A, Shapira L. IL-10 gene transfer attenuates P. gingivalis-induced inflammation. J Dent Res. 2007; 86:560–564.
Article111. Kitaura H, Fujimura Y, Yoshimatsu M, Kohara H, Morita Y, Aonuma T, Fukumoto E, Masuyama R, Yoshida N, Takano-Yamamoto T. IL-12- and IL-18-mediated, nitric oxide-induced apoptosis in TNF-α-mediated osteoclastogenesis of bone marrow cells. Calcif Tissue Int. 2011; 89:65–73.
Article112. Nagata N, Kitaura H, Yoshida N, Nakayama K. Inhibition of RANKL-induced osteoclast formation in mouse bone marrow cells by IL-12: involvement of IFN-gamma possibly induced from non-T cell population. Bone. 2003; 33:721–732.113. Kitaura H, Tatamiya M, Nagata N, Fujimura Y, Eguchi T, Yoshida N, Nakayama K. IL-18 induces apoptosis of adherent bone marrow cells in TNF-α mediated osteoclast formation in synergy with IL-12. Immunol Lett. 2006; 107:22–31.
Article114. Morita Y, Kitaura H, Yoshimatsu M, Fujimura Y, Kohara H, Eguchi T, Yoshida N. IL-18 inhibits TNF-alpha-induced osteoclastogenesis possibly via a T cell-independent mechanism in synergy with IL-12 in vivo. Calcif Tissue Int. 2010; 86:242–248.115. Dai SM, Nishioka K, Yudoh K. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Ann Rheum Dis. 2004; 63:1379–1386.116. Horwood NJ, Elliott J, Martin TJ, Gillespie MT. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol. 2001; 166:4915–4921.117. Furukawa M, Takaishi H, Takito J, Yoda M, Sakai S, Hikata T, Hakozaki A, Uchikawa S, Matsumoto M, Chiba K, et al. IL-27 abrogates receptor activator of NF-kappa B ligand-mediated osteoclastogenesis of human granulocyte-macrophage colony-forming unit cells through STAT1-dependent inhibition of c-Fos. J Immunol. 2009; 183:2397–2406.118. Kamiya S, Okumura M, Chiba Y, Fukawa T, Nakamura C, Nimura N, Mizuguchi J, Wada S, Yoshimoto T. IL-27 suppresses RANKL expression in CD4+ T cells in part through STAT3. Immunol Lett. 2011; 138:47–53.
Article119. Zolochevska O, Diaz-Quiñones AO, Ellis J, Figueiredo ML. Interleukin-27 expression modifies prostate cancer cell crosstalk with bone and immune cells in vitro. J Cell Physiol. 2013; 228:1127–1136.120. Kalliolias GD, Zhao B, Triantafyllopoulou A, Park-Min KH, Ivashkiv LB. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum. 2010; 62:402–413.121. Shukla P, Mansoori MN, Kakaji M, Shukla M, Gupta SK, Singh D. Interleukin 27 (IL-27) alleviates bone loss in estrogen-deficient conditions by induction of early growth response-2 gene. J Biol Chem. 2017; 292:4686–4699.
Article122. Kiyomiya H, Ariyoshi W, Okinaga T, Kaneuji T, Mitsugi S, Sakurai T, Habu M, Yoshioka I, Tominaga K, Nishihara T. IL-33 inhibits RANKL-induced osteoclast formation through the regulation of Blimp-1 and IRF-8 expression. Biochem Biophys Res Commun. 2015; 460:320–326.
Article123. Lima IL, Macari S, Madeira MF, Rodrigues LF, Colavite PM, Garlet GP, Soriani FM, Teixeira MM, Fukada SY, Silva TA. Osteoprotective effects of IL-33/ST2 link to osteoclast apoptosis. Am J Pathol. 2015; 185:3338–3348.
Article124. Zaiss MM, Kurowska-Stolarska M, Böhm C, Gary R, Scholtysek C, Stolarski B, Reilly J, Kerr S, Millar NL, Kamradt T, et al. IL-33 shifts the balance from osteoclast to alternatively activated macrophage differentiation and protects from TNF-alpha-mediated bone loss. J Immunol. 2011; 186:6097–6105.125. Mun SH, Oh D, Lee SK. Macrophage migration inhibitory factor down-regulates the RANKL-RANK signaling pathway by activating Lyn tyrosine kinase in mouse models. Arthritis Rheumatol. 2014; 66:2482–2493.
Article126. Mun SH, Won HY, Hernandez P, Aguila HL, Lee SK. Deletion of CD74, a putative MIF receptor, in mice enhances osteoclastogenesis and decreases bone mass. J Bone Miner Res. 2013; 28:948–959.
Article127. Gu R, Santos LL, Ngo D, Fan H, Singh PP, Fingerle-Rowson G, Bucala R, Xu J, Quinn JM, Morand EF. Macrophage migration inhibitory factor is essential for osteoclastogenic mechanisms in vitro and in vivo mouse model of arthritis. Cytokine. 2015; 72:135–145.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of NFATc1 in Osteoclast Differentiation
- Signaling Pathways in Osteoclast Differentiation
- NDRG2 Expression Decreases Tumor-Induced Osteoclast Differentiation by Down-regulating ICAM1 in Breast Cancer Cells
- Effect of Sonicated Extract of Treponema Denticola on Osteoclast Differentiation
- Regulation of matrix metalloproteinase-9 protein expression by 1alpha,25-(OH)2D3 during osteoclast differentiation