Lab Anim Res.
2017 Dec;33(4):298-307. 10.5625/lar.2017.33.4.298.
Correlation between laxative effects of uridine and suppression of ER stress in loperamide induced constipated SD rats
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang 50463, Korea. dyhwang@pusan.ac.kr
- KMID: 2402266
- DOI: http://doi.org/10.5625/lar.2017.33.4.298
Abstract
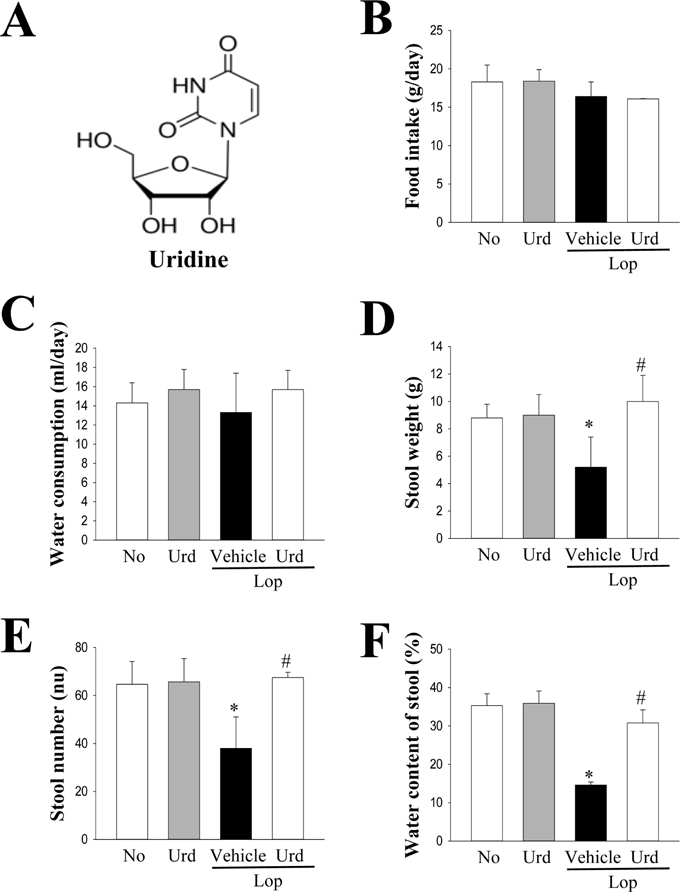
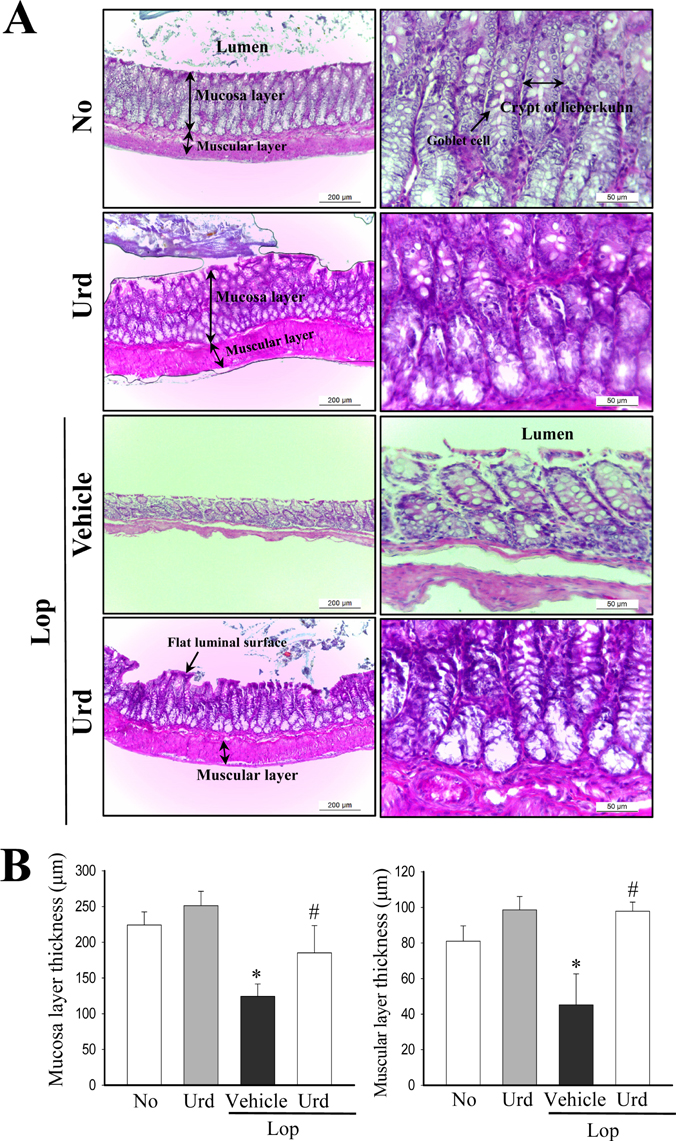
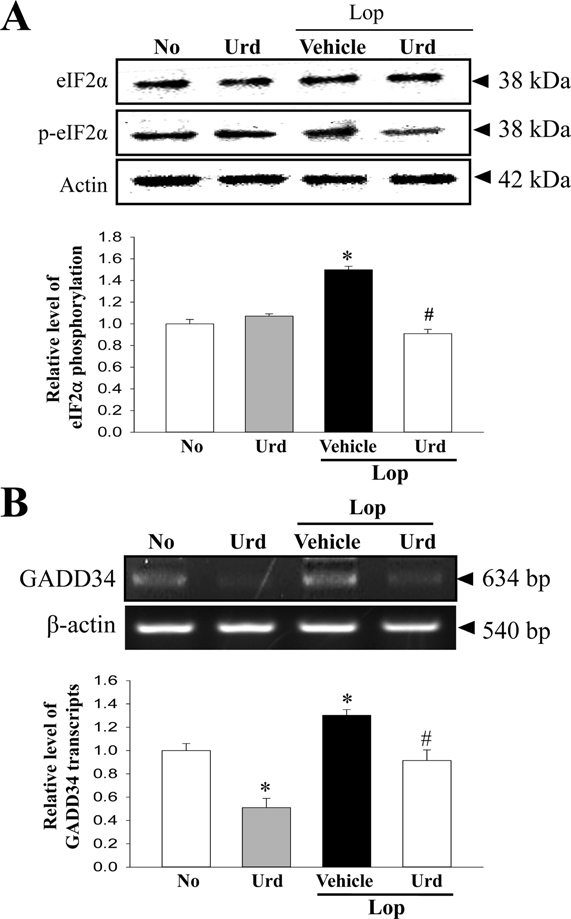
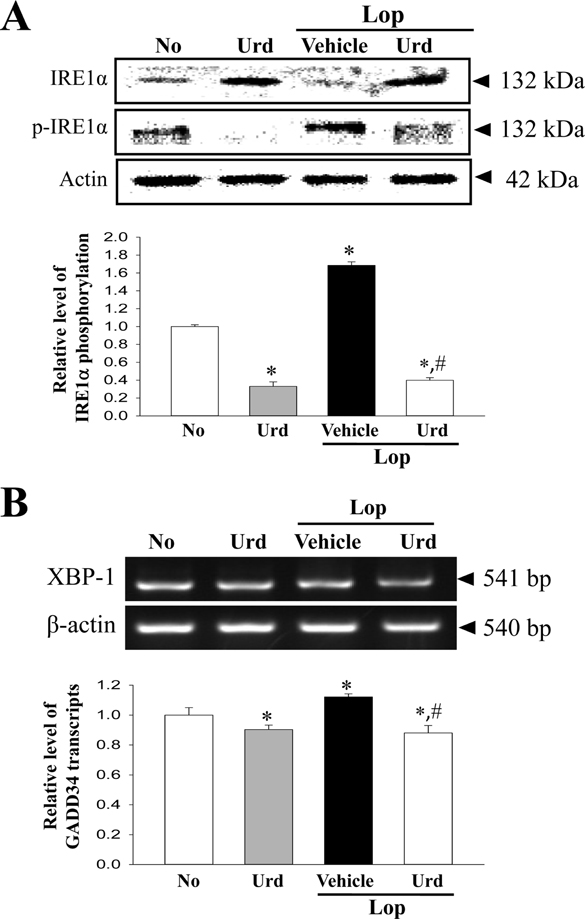
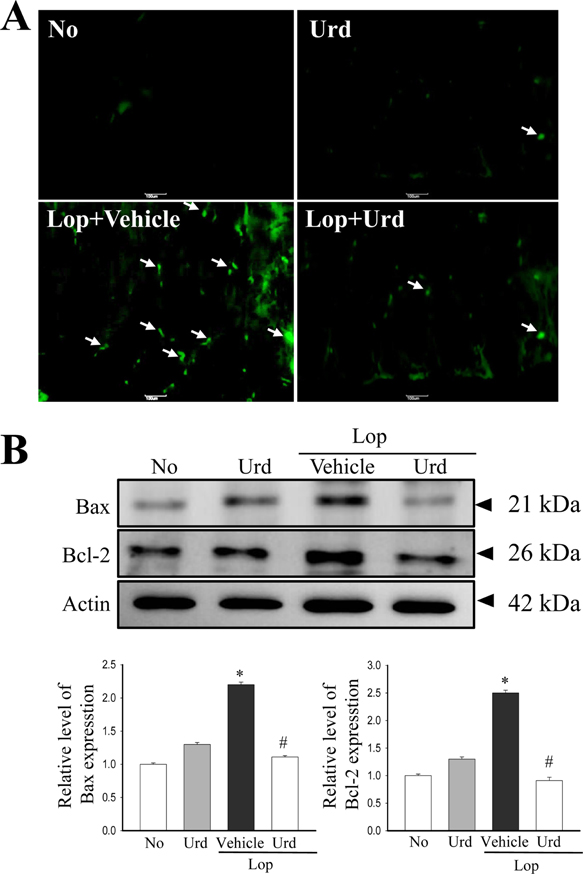
- A correlation between endoplasmic reticulum (ER) stress and laxative effects was first reported in a constipation model treated with an aqueous extract of Liriope platyphylla (AEtLP) roots. To investigate the correlation between the laxative effect of uridine (Urd) and ER stress response, alterations in the key parameters for ER stress were measured in loperamide (Lop) induced constipation Sprague Dawley (SD) rats treated with Urd. The efficacy of the laxative effect of Urd was notable on the symptoms of chronic constipation, including alteration of stool parameters and structure of the transverse colon, in Lop induced constipated SD rats. In the PERK/eIF2-ATF4 pathway of ER stress response, the levels of eukaryotic initiation factor 2 alpha (eIF2α) phosphorylation and DNA damage-inducible protein (GADD34) transcripts were significantly enhanced in the Lop+Vehicle treated group. However, the levels were restored in the Lop+Urd treated group, although few differences were detected in the decrease rate. Similar changes were observed for levels of inositol-requiring enzyme 1 beta (IRE1β) phosphorylation and X-box binding protein 1 (XBP-1) transcript in the IRE1α/XBP pathway. Furthermore, the number of ER stress-induced apoptotic cells and Bax and Bcl-2 expression were recovered in the Lop+Urd treated group compared to the Lop+Vehicle treated group. The results of the present study therefore provide first evidence that the laxative effects of Urd may be tightly correlated with the recovery of ER stress response in constipation models.
Keyword
MeSH Terms
Figure
Reference
-
1. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 5th ed. New York: Garland Science;2008. p. 723–748.2. Schonthal AH. Endoplasmic reticulum stress: Its role in disease and novel prospects for therapy. Scientifica (Cairo). 2012; 2012:857516.3. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8(7):519–529.4. Yoshida H. ER stress and disease. FEBS J. 2007; 274:630–658.5. Forman MS, Lee VMY, Trojanowski JQ. ‘Unfolding’ pathways in neurodegenerative disease. Trends Neurosci. 2003; 26(8):407–410.6. Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012; 63:317–328.7. Kim JE, Go J, Sung JE, Lee HA, Seo EJ, Yun WB, Hwang DY. Laxative effects of Liriope platyphylla are tightly correlated with suppression of endoplasmic reticulum stress in loperamide-induced constipation of SD rats. Lab Anim Res. 2016; 32(1):16–23.8. Kim JE, Go J, Sung JE, Lee HA, Yun WB, Hong JT, Hwang DY. Uridine stimulate laxative effect in the loperamide-induced constipation of SD rats through regulation of the mAChRs signaling pathway and mucin secretion. BMC Gastroenterol. 2017; 17:21.9. Kim JE, Lee YJ, Kwak MH, Ko J, Hong JT, Hwang DY. Aqueous extracts of Liriope platyphylla induced significant laxative effects on loperamide-induced constipation of SD rats. BMC Complement Altern Med. 2013; 13:333.10. Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011; 31(12):2792–2797.11. Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013; 1833(12):3460–3470.12. Doughty D. Structure and function of the gastrointestinal tract in infants and children. J Wound Ostomy Continence Nurs. 2004; 31(4):207–212.13. Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009; 337:1–35.14. Alves GA, Silva LR, Rosa EF, Aboulafia J, Freymüller-Haapalainen E, Souccar C, Nouailhetas VL. Intestine of dystrophic mice presents enhanced contractile resistance to stretching despite morphological impairment. Am J Physiol Gastrointest Liver Physiol. 2014; 306(3):G191–G199.15. Chan OT, Chiles L, Levy M, Zhai J, Yerian LM, Xu H, Xiao SY, Soffer EE, Conklin JL, Dhall D, Kahn ME, Balzer BL, Amin MB, Wang HL. Smoothelin expression in the gastrointestinal tract: implication in colonic inertia. Appl Immunohistochem Mol Morphol. 2013; 21(5):452–459.16. Tóth S, Jonecová Z, Varga J, Staško P, Kovavalèinová B, Maretta M, Veselá J. Mesenteric ischemia-reperfusion injury: specific impact on different cell populations within the jejunal wall in rats. Acta Histochem. 2012; 114(3):276–284.17. Karri S, Acosta-Martinez V, Coimbatore G. Effect of dihydrotestosterone on gastrointestinal tract of male Alzheimer's disease transgenic mice. Indian J Exp Biol. 2010; 48(5):453–465.18. Cao W, Fiocchi C, Pricolo VE. Production of IL-1beta, hydrogen peroxide, and nitric oxide by colonic mucosa decreases sigmoid smooth muscle contractility in ulcerative colitis. Am J Physiol Cell Physiol. 2005; 289(6):C1408–C1416.19. Oakes SA, Lin SS, Bassik MC. The control of endoplasmic reticulum-initiated apoptosis by the BCL-2 family of proteins. Curr Mol Med. 2006; 6:99–109.20. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001; 292:727–730.21. McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001; 21(4):1249–1259.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laxative effects of Liriope platyphylla are tightly correlated with suppression of endoplasmic reticulum stress in loperamide-induced constipation of SD rats
- Laxative effect of peanut sprout extract
- The Effect of Probiotic on Constipation in Rats
- Improvement of the intestinal epithelial barrier during laxative effects of phlorotannin in loperamide‑induced constipation of SD rats
- Regulation of gastrointestinal hormones during laxative activity of gallotannin-enriched extract isolated from Galla Rhois in loperamide-induced constipation of SD rats