Lab Anim Res.
2016 Mar;32(1):16-23. 10.5625/lar.2016.32.1.16.
Laxative effects of Liriope platyphylla are tightly correlated with suppression of endoplasmic reticulum stress in loperamide-induced constipation of SD rats
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- KMID: 2312150
- DOI: http://doi.org/10.5625/lar.2016.32.1.16
Abstract
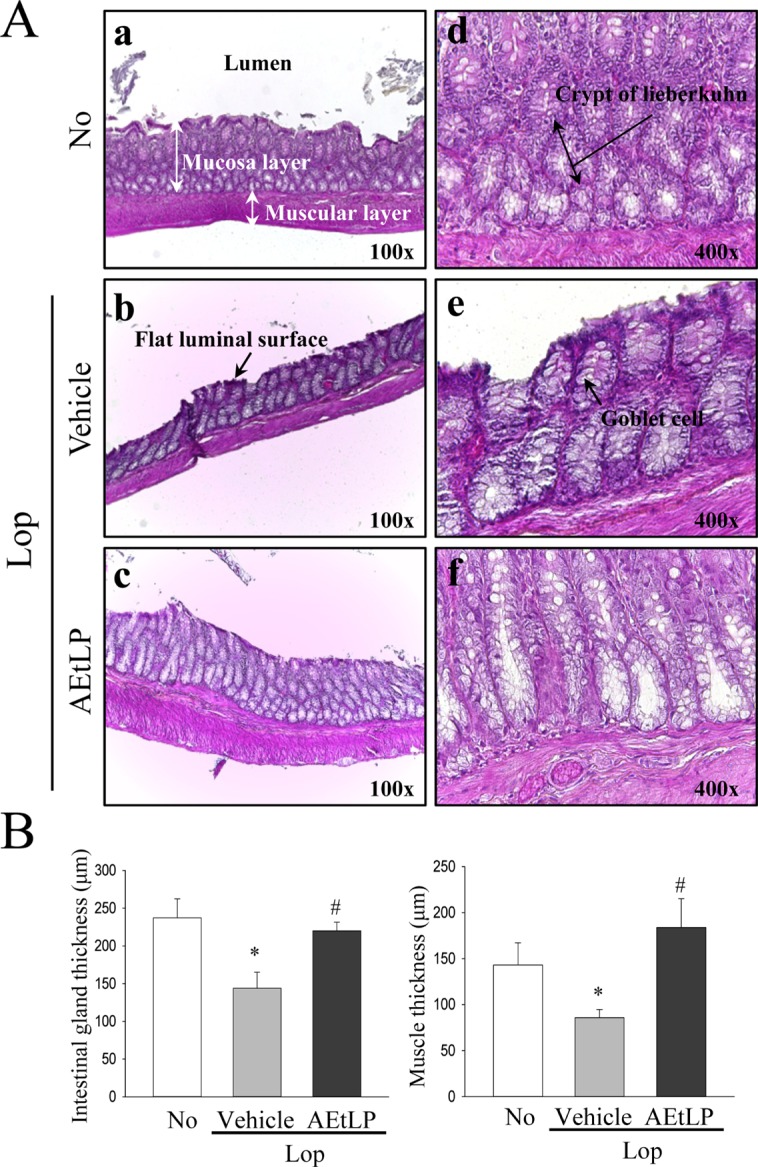
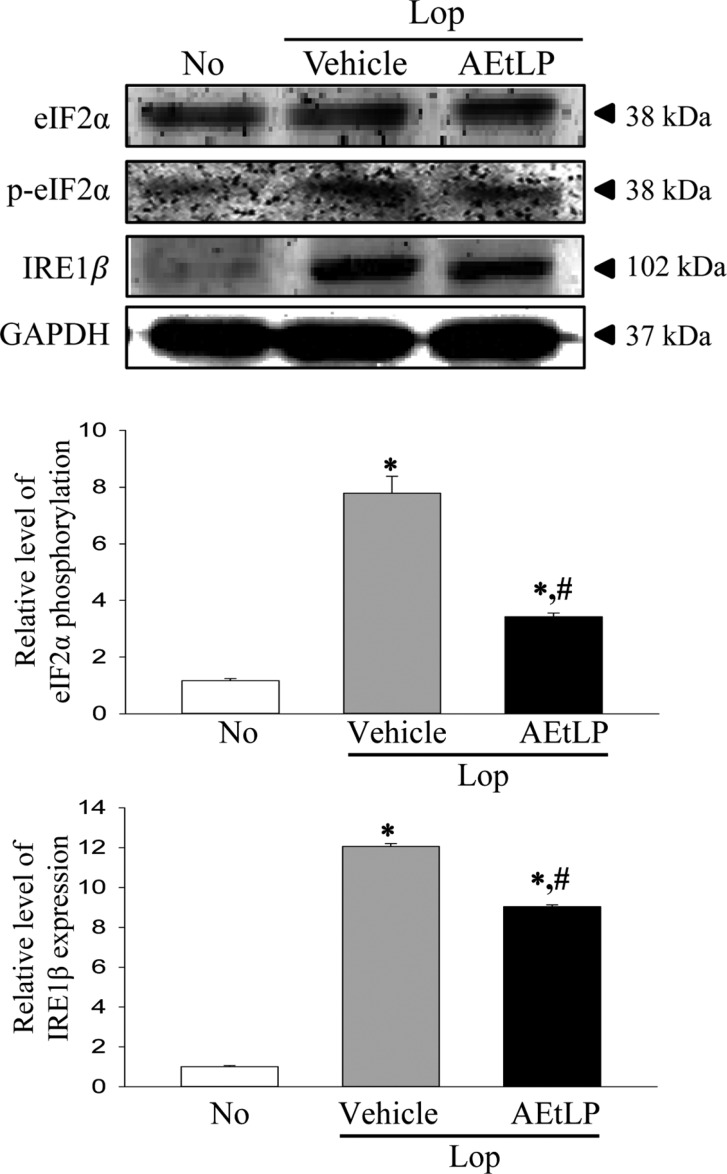
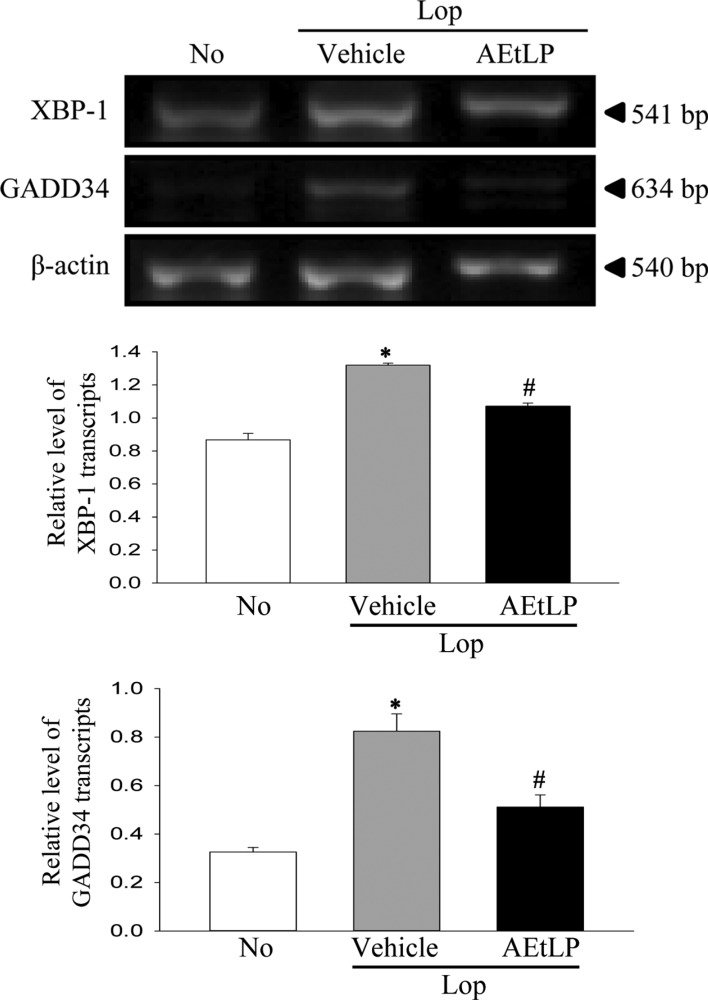
- A dysfunction of endoplasmic reticulum (ER) stress response can result in various diseases, including cancer, inflammation, diabetes and neurodegenerative disorders. To investigate whether ER stress response can play an essential role in the induction and treatment of chronic constipation, alterations in the key parameters for ER stress were measured in loperamide (Lop) induced constipation Sprague Dawley (SD) rats treated with aqueous extracts of Liriope platyphylla (AEtLP), which has been shown to have a laxative effect. Symptoms of chronic constipation including alteration of stool parameters and the transverse colon's structure were successfully induced by Lop treatment. Laxative effects such as enhancement of stools parameters, recovery of the mucosa thickness, increased muscle thickness and recovery of flat luminal surface were also observed in the Lop+AEtLP treated group. Furthermore, enhancement of eukaryotic initiation factor 2 alpha (eIF2α) phosphorylation and inositol-requiring enzyme 1 beta (IRE1β) expression, key indicators for ER stress, that were observed in the Lop+vehicle treated group were significantly recovered in the Lop+AEtLP treated group, although the phosphorylation level of c-Jun N-terminal protein kinase (JNK) remained constant. Moreover, alterations in the transcription level of the marker genes X-box binding protein 1 (XBP-1) and growth arrest and DNA damage-inducible protein (GADD34) were similar to those of eIF2α and IRE1β. However, their level was slightly or completely recovered after AEtLP treatment. Overall, this study provides the first evidence that ER stress response may be tightly correlated with chronic constipation induced by Lop treatment, as well as the laxative effects of AEtLP.
Keyword
MeSH Terms
-
Animals
Carrier Proteins
Constipation*
DNA
Endoplasmic Reticulum Stress*
Endoplasmic Reticulum*
Eukaryotic Initiation Factor-2
Inflammation
Loperamide
Mucous Membrane
Neurodegenerative Diseases
Phenobarbital
Phosphorylation
Protein Kinases
Rats*
Carrier Proteins
DNA
Eukaryotic Initiation Factor-2
Loperamide
Phenobarbital
Protein Kinases
Figure
Reference
-
1. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 4th edn. New York: Garland Science;2002.2. Schonthal AH. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo). 2012; 2012:857516. PMID: 24278747.3. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8(7):519–529. PMID: 17565364.
Article5. Forman MS, Lee VM, Trojanowski JQ. 'Unfolding' pathways in neurodegenerative disease. Trends Neurosci. 2003; 26(8):407–410. PMID: 12900170.
Article6. Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012; 63:317–328. PMID: 22248326.
Article7. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006; 313(5790):1137–1140. PMID: 16931765.
Article8. Zhang Z, Tong N, Gong Y, Qiu Q, Yin L, Lv X, Wu X. Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia-reperfusion injury. Neurosci Lett. 2011; 504(2):88–92. PMID: 21939735.
Article9. Kim I, Shu CW, Xu W, Shiau CW, Grant D, Vasile S, Cosford ND, Reed JC. Chemical biology investigation of cell death pathways activated by endoplasmic reticulum stress reveals cytoprotective modulators of ASK1. J Biol Chem. 2009; 284(3):1593–1603. PMID: 19004820.
Article10. Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005; 307(5711):935–939. PMID: 15705855.11. Appierto V, Tiberio P, Villani MG, Cavadini E, Formelli F. PLAB induction in fenretinide-induced apoptosis of ovarian cancer cells occurs via a ROS-dependent mechanism involving ER stress and JNK activation. Carcinogenesis. 2009; 30(5):824–831. PMID: 19325135.
Article12. Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci U S A. 2006; 103(3):768–773. PMID: 16407140.13. Wang XZ, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science. 1996; 272(5266):1347–1349. PMID: 8650547.
Article14. Kim JE, Lee YJ, Kwak MH, Ko J, Hong JT, Hwang DY. Aqueous extracts of Liriope platyphylla induced significant laxative effects on loperamide-induced constipation of SD rats. BMC Complement Altern Med. 2013; 13:333. PMID: 24274470.
Article15. Kim HJ, Kim J, Kim SJ, Lee SH, Park YS, Park BK, Kim BS, Kim SK, Cho SD, Jung JW, Nam JS, Choi CS, Jung JY. Anti-inflammatory effect of quercetin on picryl chloride-induced contact dermatitis in BALB/c mice. Lab Anim Res. 2010; 26(1):7–13.
Article16. Kim JE, Hwang IS, Choi SI, Lee HR, Lee YJ, Goo JS, Lee HS, Son HJ, Jang MJ, Lee SH, Kang BC, Hwang DY. Aqueous extract of Liriope platyphylla, a traditional Chinese medicine, significantly inhibits abdominal fat accumulation and improves glucose regulation in OLETF type II diabetes model rats. Lab Anim Res. 2012; 28(3):181–191. PMID: 23091518.17. Wintola OA, Sunmonu TO, Afolayan AJ. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010; 10:95. PMID: 20723249.
Article18. Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS, Li B, Lee GH, Sung MS, Ha KC, Back HI, Kim SY, Park SH, Oh MR, Kim MG, Jeon JY, Im YJ, Hwang MH, So BO, Shin SJ, Yoo WH, Kim HR, Chae HJ, Chae SW. Effects of Ficus carica paste on loperamide-induced constipation in rats. Food Chem Toxicol. 2012; 50(3-4):895–902. PMID: 22178225.19. Leung FW. Etiologic factors of chronic constipation: review of the scientific evidence. Dig Dis Sci. 2007; 52(2):313–316. PMID: 17219073.20. Kim JE, Lee YJ, Kwak MH, Jun G, Koh EK, Song SH, Seong JE, Kim JW, Kim KB, Kim S, Hwang DY. Metabolomics approach to serum biomarker for loperamide-induced constipation in SD rats. Lab Anim Res. 2014; 30(1):35–43. PMID: 24707303.
Article21. Carrington EV, Evers J, Grossi U, Dinning PG, Scott SM, O'Connell PR, Jones JF, Knowles CH. A systematic review of sacral nerve stimulation mechanisms in the treatment of fecal incontinence and constipation. Neurogastroenterol Motil. 2014; 26(9):1222–1237. PMID: 25167953.
Article22. Gyawali B, Hayashi N, Tsukuura H, Honda K, Shimokata T, Ando Y. Opioid-induced constipation. Scand J Gastroenterol. 2015; 50(11):1331–1338. PMID: 26061717.
Article23. Li Y, Yu Z, Xu B. Mechanism progress on enteric nervous system of acupuncture for slow transit constipation. Zhongguo Zhen Jiu. 2015; 35(3):309–312. PMID: 26062213.24. Awouters F, Niemegeers CJ, Janssen PA. Pharmacology of antidiarrheal drugs. Annu Rev Pharmacol Toxicol. 1983; 23:279–301. PMID: 6307123.
Article25. Church J, Fletcher EJ, Abdel-Hamid K, MacDonald JF. Loperamide blocks high-voltage-activated calcium channels and N-methyl-D-aspartate-evoked responses in rat and mouse cultured hippocampal pyramidal neurons. Mol Pharmacol. 1994; 45(4):747–757. PMID: 8183255.26. Reynolds IJ, Gould RJ, Snyder SH. Loperamide: blockade of calcium channels as a mechanism for antidiarrheal effects. J Pharmacol Exp Ther. 1984; 231(3):628–632. PMID: 6502516.27. Burleigh DE. Opioid and non-opioid actions of loperamide on cholinergic nerve function in human isolated colon. Eur J Pharmacol. 1988; 152(1-2):39–46. PMID: 2850201.
Article28. Nagai K, Chiba A, Nishino T, Kubota T, Kawagishi H. Dilinoleoyl-phosphatidylethanolamine from Hericium erinaceum protects against ER stress-dependent Neuro2a cell death via protein kinase C pathway. J Nutr Biochem. 2006; 17(8):525–530. PMID: 16426828.
Article29. Shaerzadeh F, Alamdary SZ, Esmaeili MA, Sarvestani NN, Khodagholi F. Neuroprotective effect of Salvia sahendica is mediated by restoration of mitochondrial function and inhibition of endoplasmic reticulum stress. Neurochem Res. 2011; 36(12):2216–2226. PMID: 21769643.30. Tanaka J, Nakanishi T, Shimoda H, Nakamura S, Tsuruma K, Shimazawa M, Matsuda H, Yoshikawa M, Hara H. Purple rice extract and its constituents suppress endoplasmic reticulum stress-induced retinal damage in vitro and in vivo. Life Sci. 2013; 92(1):17–25. PMID: 23123597.31. Lee J, Hong SW, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Lee WY. Exendin-4 attenuates endoplasmic reticulum stress through a SIRT1-dependent mechanism. Cell Stress Chaperones. 2014; 19(5):649–656. PMID: 24446069.
Article32. Chiu SC, Huang SY, Chen SP, Su CC, Chiu TL, Pang CY. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis. 2013; 16(4):315–322. PMID: 24042854.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between laxative effects of uridine and suppression of ER stress in loperamide induced constipated SD rats
- Metabolomics approach to serum biomarker for laxative effects of red Liriope platyphylla in loperamide-induced constipation of SD rats
- Laxative effect of peanut sprout extract
- Regulation of gastrointestinal hormones during laxative activity of gallotannin-enriched extract isolated from Galla Rhois in loperamide-induced constipation of SD rats
- The Effect of Probiotic on Constipation in Rats