Lab Anim Res.
2018 Dec;34(4):223-231. 10.5625/lar.2018.34.4.223.
Regulation of gastrointestinal hormones during laxative activity of gallotannin-enriched extract isolated from Galla Rhois in loperamide-induced constipation of SD rats
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- KMID: 2459299
- DOI: http://doi.org/10.5625/lar.2018.34.4.223
Abstract
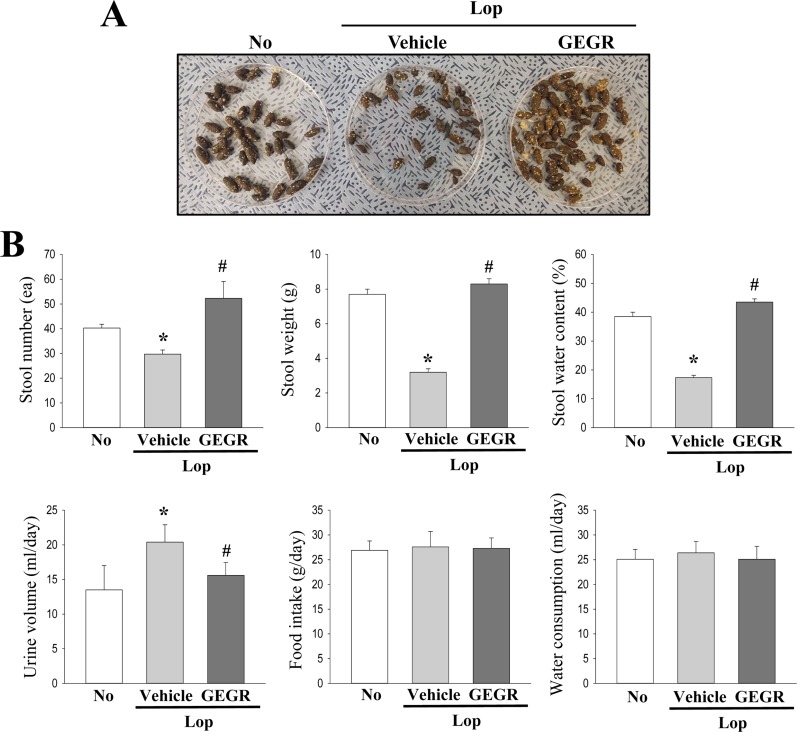
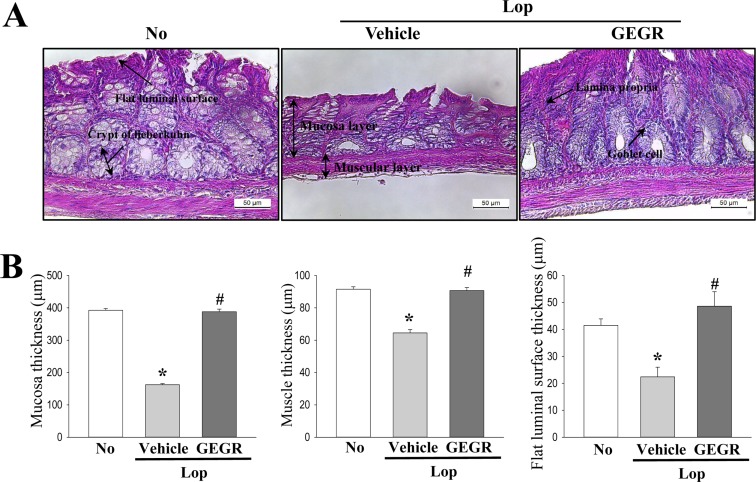
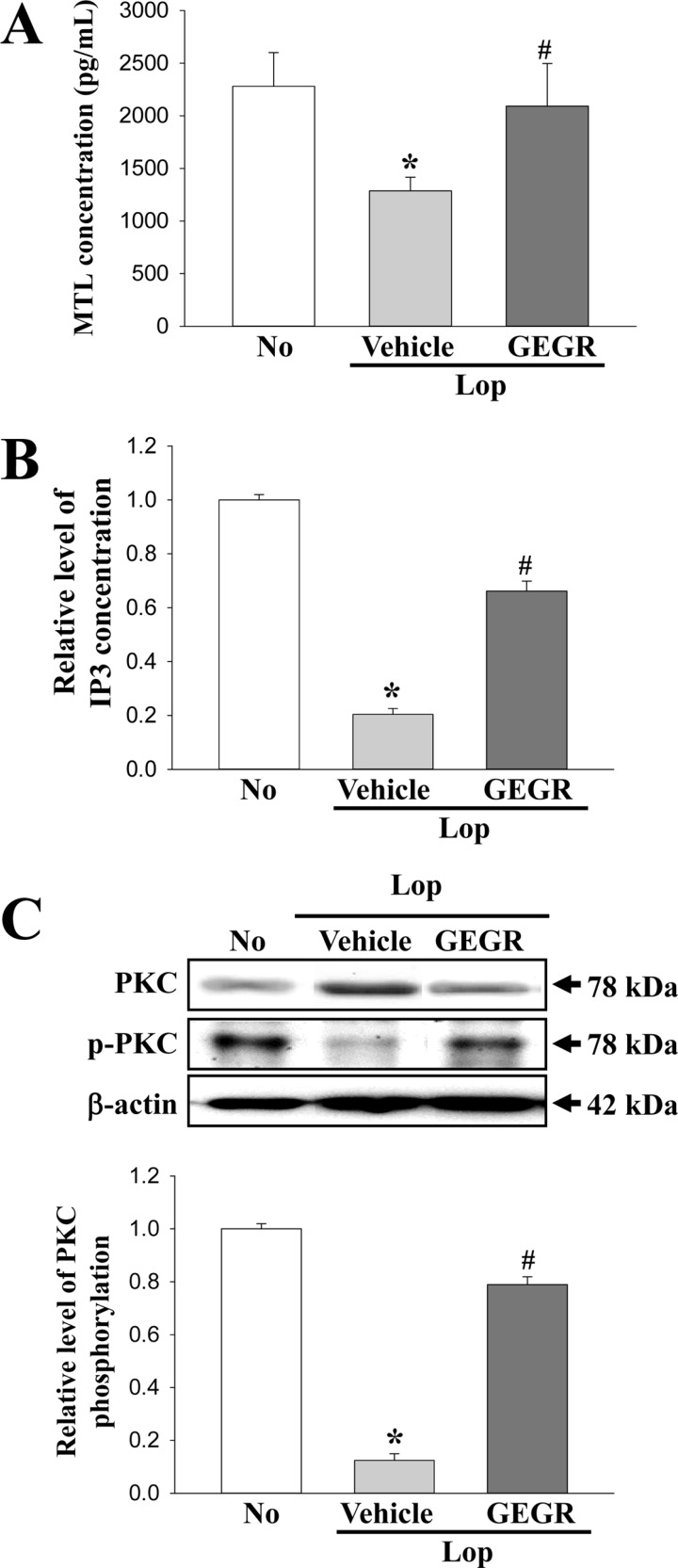
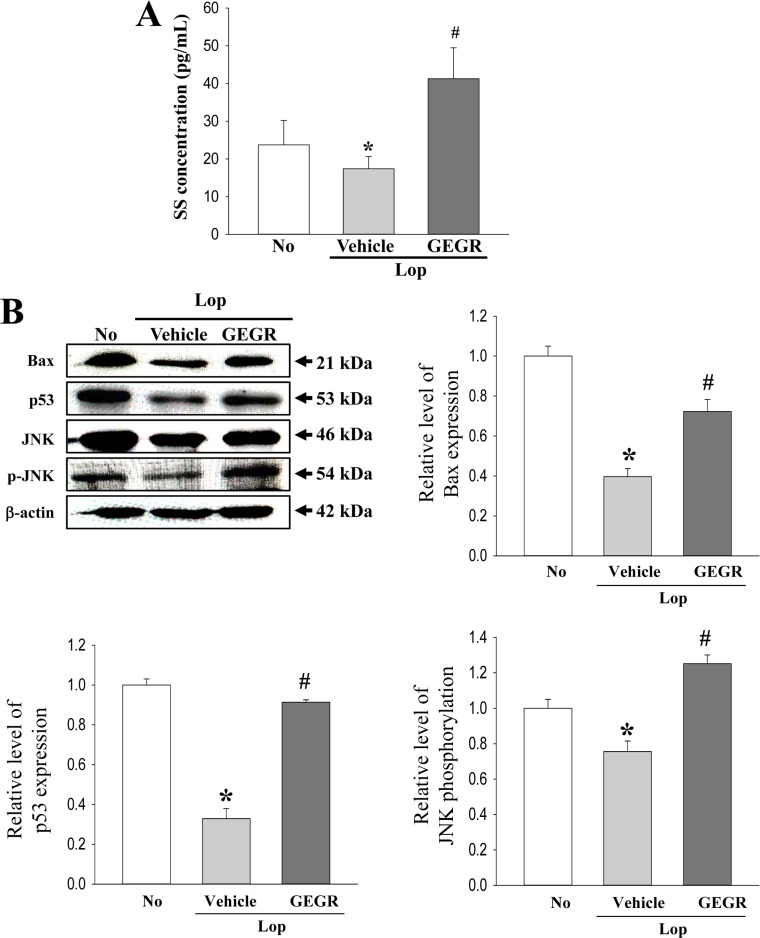
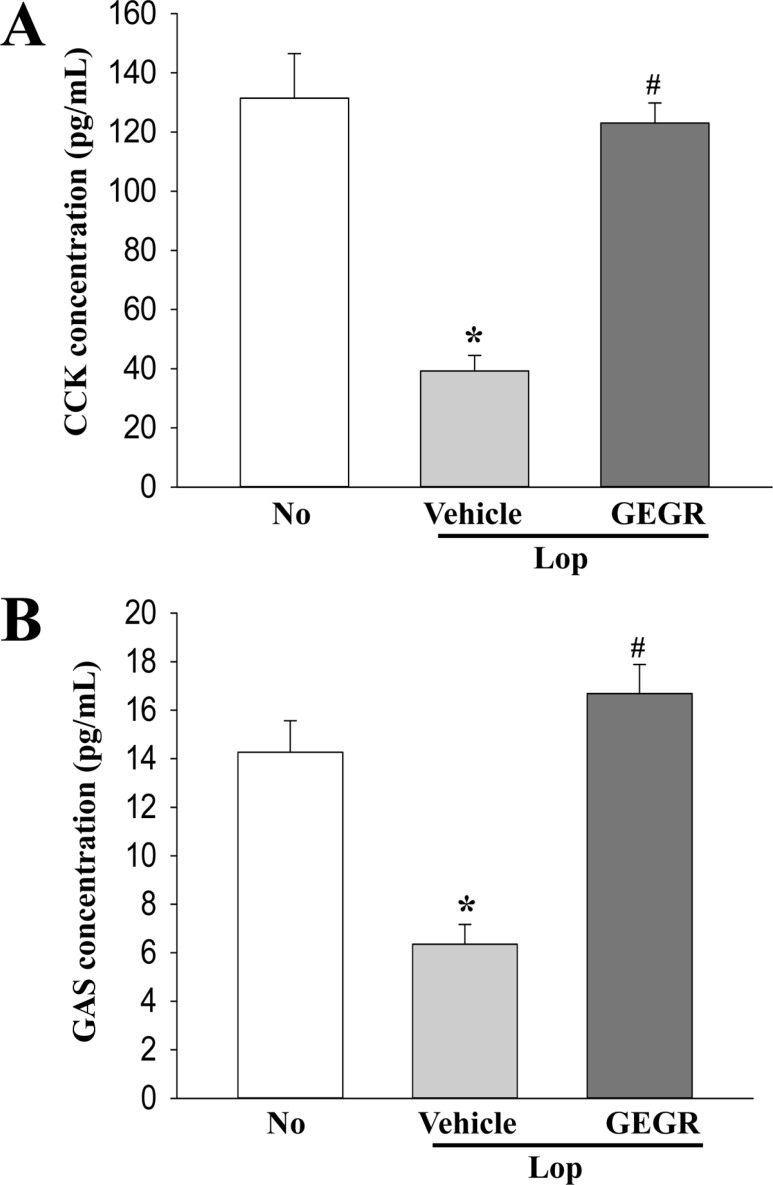
- Regulation of gastrointestinal hormones have been reported in animal models for constipation undergoing laxative therapy when administered herbal products. We undertook to investigate whether the laxative activity of gallotannin-enriched extracts isolated from Galla Rhois (GEGR) affects the regulation of gastrointestinal hormones, by examining the concentration of four hormones and the activation of their receptors in the loperamide (Lop)-induced constipation model. Stool parameters, including number, weight and water content, were significantly recovered in the Lop+GEGR treated group, relative to the Lop+vehicle treated group; however, food intake and water consumption were maintained at a constant level. Also, a similar recovery was detected for thickness of mucosa, muscle and flat luminal surface in the Lop+GEGR treated group. Furthermore, concentration of the four gastrointestinal hormones evaluated, namely, cholecystokinin (CCK), gastrin (GAS), somatostatin (SS) and motilin (MTL), were lower in the Lop+vehicle treated group than the No treated group, but were remarkably enhanced in the Lop+GEGR treated group. Moreover, the downstream signaling pathway of MTL and SS receptors were recovered after GEGR administration. Results of the present study therefore indicate that the laxative effects of GEGR treatment may be tightly related with the regulation of gastrointestinal hormones in the Lop-induced constipation model.
MeSH Terms
Figure
Reference
-
1. Pare P. The approach to diagnosis and treatment of chronic constipation: suggestions for a general practitioner. Can J Gastroenterol. 2011; 25(Suppl B):36B–40B.
Article2. Lacy BE, Levenick JM, Crowell M. Chronic constipation: new diagnostic and treatment approaches. Therap Adv Gastroenterol. 2012; 5(4):233–247.
Article3. El-Salhy M, Svensen R, Hatlebakk JG, Gilja OH, Hausken T. Chronic constipation and treatment options (Review). Mol Med Rep. 2014; 9(1):3–8. PMID: 24189940.
Article4. Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS, Li B, Lee GH, Sung MS, Ha KC, Back HI, Kim SY, Park SH, Oh MR, Kim MG, Jeon JY, Im YJ, Hwang MH, So BO, Shin SJ, Yoo WH, Kim HR, Chae HJ, Chae SW. Effects of Ficus carica paste on loperamide-induced constipation in rats. Food Chem Toxicol. 2012; 50(3-4):895–902. PMID: 22178225.5. Méité S, Bahi C, Yéo D, Datté JY, Djaman JA, N'guessan DJ. Laxative activities of Mareya micrantha (Benth.) Müll. Arg.(Euphorbiaceae) leaf aqueous extract in rats. BMC Complement Altern Med. 2010; 10:7. PMID: 20158903.
Article6. Kim JE, Lee YJ, Kwak MH, Ko J, Hong JT, Hwang DY. Aqueous extracts of Liriope platyphylla induced significant laxative effects on loperamide-induced constipation of SD rats. BMC Complement Altern Med. 2013; 13:333. PMID: 24274470.
Article7. Kim JE, Go J, Koh EK, Song SH, Sung JE, Lee HA, Lee YH, Hong JT, Hwang DY. Gallotannin-enriched extract isolated from Galla Rhois may be a functional candidate with laxative effects for treatment of loperamide-induced constipation of SD rats. PLoS One. 2016; 9. 12. 11(9):e0161144. PMID: 27618438.
Article8. Liu Y, Zhao XR, Wang R, Qiu GQ, Zhang M. Effect of Zhizhuwan on gastrointestinal peptide concentrations in plasma of diabetic gastroenteropathy with constipation patients. Zhongguo Zhong Yao Za Zhi. 2008; 33(24):2966–2968. PMID: 19294863.9. Aydin S, Donder E, Akin OK, Sahpaz F, Kendir Y, Alnema MM. Fat-free milk as a therapeutic approach for constipation and the effect on serum motilin and ghrelin levels. Nutrition. 2010; 26(10):981–985. PMID: 20303236.
Article10. Zhang C, Guo L, Guo X, Li G, Guo X. Short and long-term efficacy of combining Fuzhengliqi mixture with acupuncture in treatment of functional constipation. J Tradit Chin Med. 2013; 33(1):51–59. PMID: 23596812.11. Zhao X, Qian Y, Suo H, Du M, Li G, Liu Z, Li J. Preventive effect of Lactobacillus fermentum Zhao on activated carbon-induced constipation in mice. J Nutr Sci Vitaminol (Tokyo). 2015; 61(2):131–137. PMID: 26052143.12. Luo D, Qu C, Zhang Z, Xie J, Xu L, Yang H, Li C, Lin G, Wang H, Su Z. Granularity and laxative effect of ultrafine powder of Dendrobium officinale. J Med Food. 2017; 20(2):180–188. PMID: 28146409.13. Yin J, Liang Y, Wang D, Yan Z, Yin H, Wu D, Su Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int J Mol Med. 2018; 41(2):649–658. PMID: 29207043.
Article14. Lee YH, Hwang EK, Kim HD. Colorimetric assay and antibacterial activity of cotton, silk, and wool fabrics dyed with peony, pomegranate, clove, Coptis chinenis and gallnut extracts. Materials. 2009; 2(1):10–21.15. Lee YH, Hwang EK, Baek YM, Kim HD. Deodorizing function and antibacterial activity of fabrics dyed with gallnut (Galla Chinensis) extract. Text Res J. 2015; 85(10):1045–1054.16. Wintola OA, Sunmonu TO, Afolayan AJ. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010; 10:95. PMID: 20723249.
Article17. Redfern JS, O'Dorisio TM. Therapeutic uses of gastrointestinal peptides. Endocrinol Metab Clin North Am. 1993; 22(4):845–873. PMID: 8125075.
Article18. Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005; 128(1):175–191. PMID: 15633135.
Article19. Nauck MA. Unraveling the science of incretin biology. Eur J Intern Med. 2009; 122(Suppl 2):S3–S10.
Article20. Imperiale TF, Birgisson S. Somatostatin or octreotide compared with H2 antagonists and placebo in the management of acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis. Ann Intern Med. 1997; 127(12):1062–1071. PMID: 9412308.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antifungal Activity of the Extracts from Galla rhois against Candida albicans
- Laxative effect of peanut sprout extract
- Correlation between laxative effects of uridine and suppression of ER stress in loperamide induced constipated SD rats
- Identification and partial purification of antibacterial compounds against Streptococcus mutans from Galla Rhois
- Improvement of the intestinal epithelial barrier during laxative effects of phlorotannin in loperamide‑induced constipation of SD rats