Korean J Neurotrauma.
2017 Oct;13(2):76-84. 10.13004/kjnt.2017.13.2.76.
Ganoderma Lucidum Protects Rat Brain Tissue Against Trauma-Induced Oxidative Stress
- Affiliations
-
- 1Department of Neurosurgery, Faculty of Medicine, Dicle University, Diyarbakır, Turkey.
- 2Department of Medical Biology, Faculty of Medicine, Dicle University, Diyarbakır, Turkey.
- 3Department of Histology and Embryology, Faculty of Medicine, Dicle University, Diyarbakır, Turkey. firatasir@gmail.com
- 4Ataturk Health Hıgh School, Dicle University, Diyarbakır, Turkey.
- KMID: 2394538
- DOI: http://doi.org/10.13004/kjnt.2017.13.2.76
Abstract
OBJECTIVE
Traumatic brain injury causes tissue damage, breakdown of cerebral blood flow and metabolic regulation. This study aims to investigate the protective influence of antioxidant Ganoderma lucidum (G. lucidum) polysaccharides (GLPs) on brain injury in brain-traumatized rats.
METHODS
Sprague-Dawley conducted a head-traumatized method on rats by dropping off 300 g weight from 1 m height. Groups were categorized as control, G. lucidum, trauma, trauma+ G. lucidum (20 mL/kg per day via gastric gavage). Brain tissues were dissected from anesthetized rats 7 days after injury. For biochemical analysis, malondialdehyde, glutathione and myeloperoxidase values were measured.
RESULTS
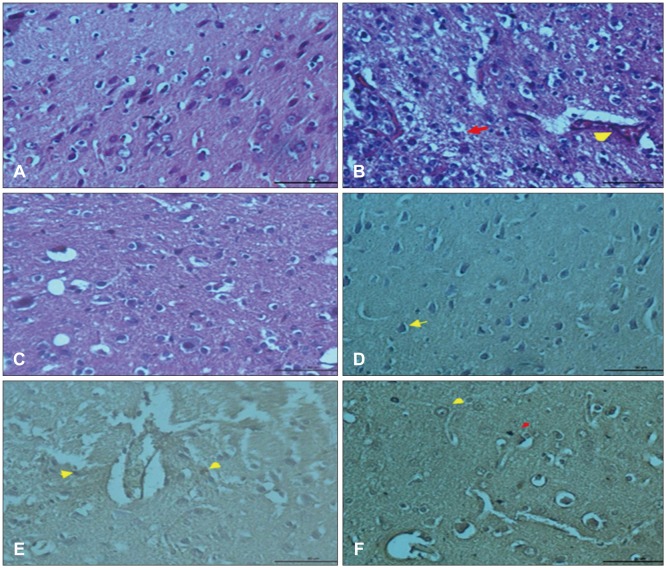
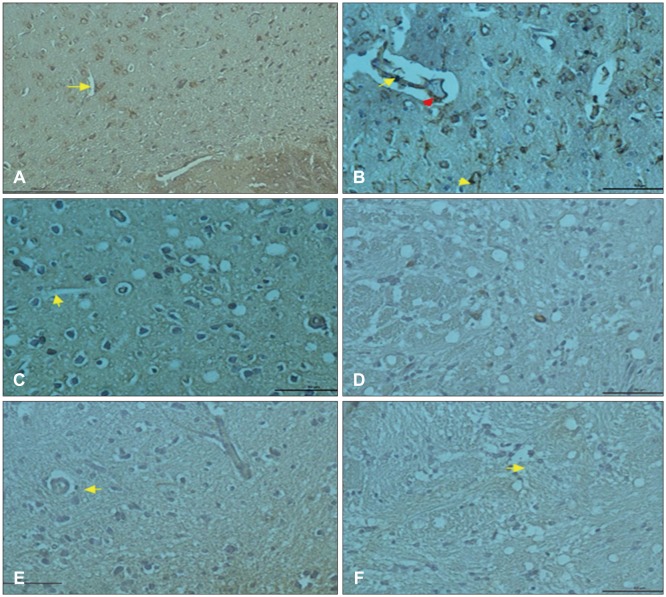
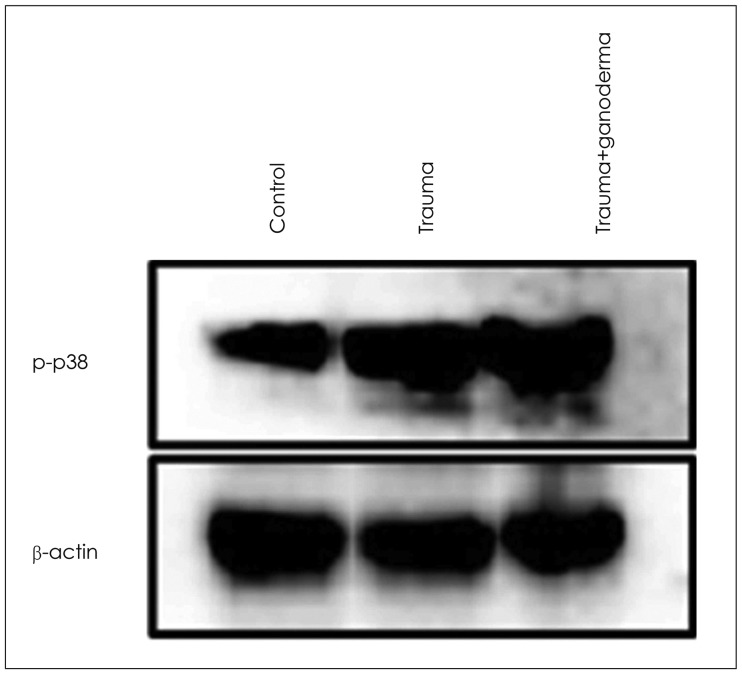
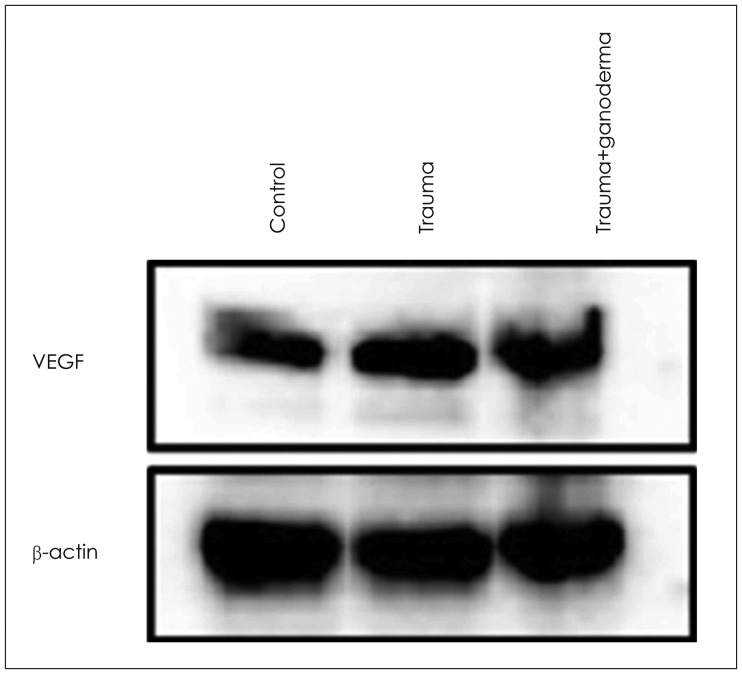
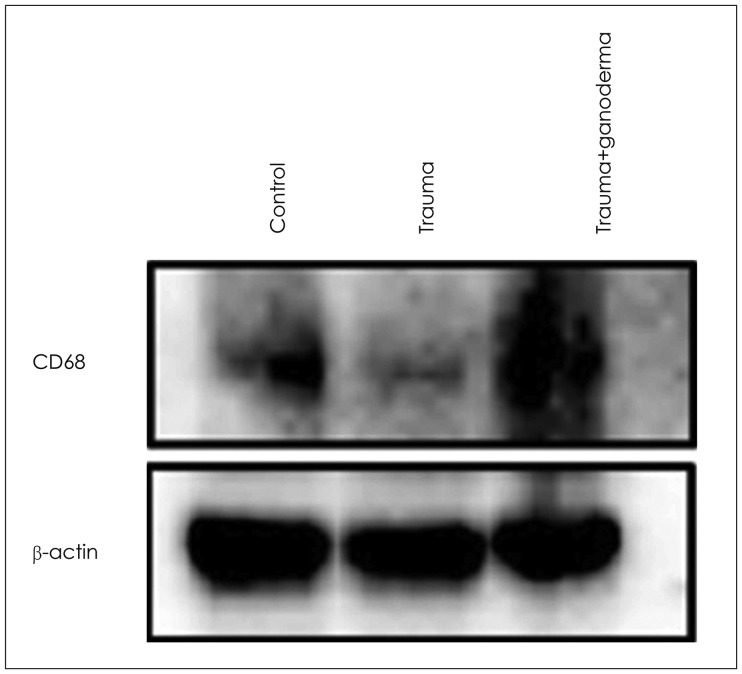
In histopathological examination, neuronal damage in brain cortex and changes in blood brain barrier were observed. In the analysis of immunohistochemical and western blot, p38 mitogen-activated protein kinase, vascular endothelial growth factor and cluster of differentiation 68 expression levels were shown. These analyzes demonstrated the beneficial effects of GLPs on brain injury.
CONCLUSION
We propose that GLPs treatment after brain injury could be an alternative treatment to decraseing inflammation and edema, preventing neuronal and glial cells degeneration if given in appropriate dosage and in particular time intervals.
Keyword
MeSH Terms
-
Animals
Blood-Brain Barrier
Blotting, Western
Brain Injuries
Brain*
Cerebrovascular Circulation
Edema
Ganoderma*
Glutathione
Inflammation
Malondialdehyde
Methods
Neuroglia
Neurons
Oxidative Stress*
Peroxidase
Polysaccharides
Protein Kinases
Rats*
Rats, Sprague-Dawley
Reishi*
Vascular Endothelial Growth Factor A
Glutathione
Malondialdehyde
Peroxidase
Polysaccharides
Protein Kinases
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Ayata C, Ropper AH. Ischaemic brain oedema. J Clin Neurosci. 2002; 9:113–124. PMID: 11922696.
Article2. Cheung WM, Hui WS, Chu PW, Chiu SW, Ip NY. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000; 486:291–296. PMID: 11119721.
Article3. Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma. 1994; 11:499–506. PMID: 7861443.
Article4. de Lores Arnaiz GR, Ordieres MG. Brain Na(+), K(+)-ATPase activity In aging and disease. Int J Biomed Sci. 2014; 10:85–102. PMID: 25018677.5. Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000; 62:649–671. PMID: 10880854.
Article6. Gao Y, Zhou S, Wen J, Huang M, Xu A. Mechanism of the antiulcerogenic effect of Ganoderma lucidum polysaccharides on indomethacin-induced lesions in the rat. Life Sci. 2002; 72:731–745. PMID: 12467913.
Article7. Gentleman SM, Leclercq PD, Moyes L, Graham DI, Smith C, Griffin WS, et al. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004; 146:97–104. PMID: 15542269.
Article8. Hakan T, Toklu HZ, Biber N, Ozevren H, Solakoglu S, Demirturk P, et al. Effect of COX-2 inhibitor meloxicam against traumatic brain injury-induced biochemical, histopathological changes and blood-brain barrier permeability. Neurol Res. 2010; 32:629–635. PMID: 19660237.
Article9. Hall R, Murdoch J. Brain protection: physiological and pharmacological considerations. Part II: The pharmacology of brain protection. Can J Anaesth. 1990; 37:762–777. PMID: 2225293.
Article10. Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990; 24:285–295. PMID: 1963456.
Article11. Hu ZL, Wen SG, Yu RJ, Zhu Y. Effects of Ganoderma lucidum fungus mixtureon immune enhancement in mice. Shandong Zhongyiyao Daxue Xuebao. 2003; 27:683–687.12. Ji Z, Tang Q, Zhang J, Yang Y, Jia W, Pan Y. Immunomodulation of RAW264.7 macrophages by GLIS, a proteopolysaccharide from Ganoderma lucidum. J Ethnopharmacol. 2007; 112:445–450. PMID: 17524580.
Article13. Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013; 136:28–42. PMID: 23365092.
Article14. Jones S, Janardhanan KK. Antioxidant and antitumor activity of ganoderma lucidum (Curt.: Fr.) P. Karst.-Reishi (Aphyllophoromycetideae) from South India. Int J Med Mushrooms. 2000; 2:195–200.
Article15. Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009; 29:13435–13444. PMID: 19864556.
Article16. Krum JM, Khaibullina A. Inhibition of endogenous VEGF impedes revascularization and astroglial proliferation: roles for VEGF in brain repair. Exp Neurol. 2003; 181:241–257. PMID: 12781997.
Article17. Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012; 26:1191–1201. PMID: 22728326.
Article18. Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013; 34:1397–1411. PMID: 23273602.
Article19. Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008; 16:154–164. PMID: 18253055.
Article20. Lakshmi B, Ajith TA, Sheena N, Gunapalan N, Janardhanan KK. Antiperoxidative, anti-inflammatory, and antimutagenic activities of ethanol extract of the mycelium of Ganoderma lucidum occurring in South India. Teratog Carcinog Mutagen. 2003; Suppl 1:85–97.21. Li JM, Zhao YN, Chen CX, Li SX. Ischemic preconditioning on the sand mouse ischemia-reperfusion injury p38MAPK activation function. Zhong Hua Shen Jing Wai Ke Za Zhi. 2011; 27:741–745.22. Lin ZB, Zhang HN. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol Sin. 2004; 25:1387–1395. PMID: 15525457.23. Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005; 25:29–38. PMID: 15539615.
Article24. Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994; 80:291–300. PMID: 8283269.25. Mizushina Y, Takahashi N, Hanashima L, Koshino H, Esumi Y, Uzawa J, et al. Lucidenic acid O and lactone, new terpene inhibitors of eukaryotic DNA polymerases from a basidiomycete, Ganoderma lucidum. Bioorg Med Chem. 1999; 7:2047–2052. PMID: 10530954.
Article26. Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008; 88:1243–1276. PMID: 18923182.
Article27. Schreibelt G, van Horssen J, van Rossum S, Dijkstra CD, Drukarch B, de Vries HE. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res Rev. 2007; 56:322–330. PMID: 17761296.
Article28. Shi YE, Wen RS, Cao XL, Wang RB, Wang XG. Effect of Ganoderma lucidum rich with selenium on the brain ischemia in rat. J Hebei Chinese Drug. 1998; 4:27–28.29. Shibuya M. Brain angiogenesis in developmental and pathological processes: therapeutic aspects of vascular endothelial growth factor. Febs j. 2009; 276:4636–4643. PMID: 19664071.
Article30. Sköld MK, von Gertten C, Sandberg-Nordqvist AC, Mathiesen T, Holmin S. VEGF and VEGF receptor expression after experimental brain contusion in rat. J Neurotrauma. 2005; 22:353–367. PMID: 15785231.
Article31. Smith C, Gentleman SM, Leclercq PD, Murray LS, Griffin WS, Graham DI, et al. The neuroinflammatory response in humans after traumatic brain injury. Neuropathol Appl Neurobiol. 2013; 39:654–666. PMID: 23231074.
Article32. Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004; 76:509–513. PMID: 15218057.
Article33. Tang Z, Liao Z, Shi Q, Xie Y, He Z, Zhan Y. Blocking p38 signal pathway lowers MMP-9 expression and reduces brain edema in rats with traumatic brain injury. Nan Fang Yi Ke Da Xue Xue Bao. 2012; 32:928–931. PMID: 22820569.34. Ucar T, Tanriover G, Gurer I, Onal MZ, Kazan S. Modified experimental mild traumatic brain injury model. J Trauma. 2006; 60:558–565. PMID: 16531854.
Article35. Wang ML, Huang XJ, Fang SH, Yuan YM, Zhang WP, Lu YB, et al. Leukotriene D4 induces brain edema and enhances CysLT2 receptor-mediated aquaporin 4 expression. Biochem Biophys Res Commun. 2006; 350:399–404. PMID: 17010308.
Article36. Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS, Shiao MS, et al. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer. 1997; 70:699–705. PMID: 9096652.
Article37. Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Crit Rev Immunol. 1999; 19:65–96. PMID: 9987601.
Article38. Wittko-Schneider IM, Schneider FT, Plate KH. Brain homeostasis: VEGF receptor 1 and 2-two unequal brothers in mind. Cell Mol Life Sci. 2013; 70:1705–1725. PMID: 23475067.
Article39. Zhang Z, Zhang ZY, Wu Y, Schluesener HJ. Lesional accumulation of CD163+ macrophages/microglia in rat traumatic brain injury. Brain Res. 2012; 1461:102–110. PMID: 22583855.
Article40. Zhao HB, Lin SQ, Liu JH, Lin ZB. Polysaccharide extract isolated from ganoderma lucidum protects rat cerebral cortical neurons from hypoxia/reoxygenation injury. J Pharmacol Sci. 2004; 95:294–298. PMID: 15215656.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Occupational asthma induced by ganoderma spores
- Comparison of Characteristics of Ganoderma lucidum According to Geographical Origins : Consideration of Growth Characteristics(I)
- Comparison of Characteristics of Ganoderma lucidum According to Geographical Origins : Consideration of Morphological Characteristics(II)
- Taxonomic Position and Species Identity of the Cultivated Yeongji 'Ganoderma lucidum' in Korea
- Taxonomy of Ganoderma lucidum from Korea Based on rDNA and Partial beta-Tubulin Gene Sequence Analysis