Infect Chemother.
2017 Sep;49(3):205-212. 10.3947/ic.2017.49.3.205.
Safety and Efficacy of Ziagen (Abacavir Sulfate) in HIV-Infected Korean Patients
- Affiliations
-
- 1GlaxoSmithKline Korea, Seoul, Korea.
- 2Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea. ksw2kms@knu.ac.kr
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea.
- 5Division of Infectious Diseases, Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea.
- 6Division of Infectious Diseases, Department of Internal Medicine, Chungnam National University School of Medicine, Daejon, Korea.
- 7Department of Infectious Disease, Chonnam National University Medical School, Gwangju, Korea.
- 8Department of Internal Medicine, Wonju College of Medicine, Yonsei University, Wonju, Korea.
- 9Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Chosun University School of Medicine, Gwangju, Korea.
- 11Department of Internal Medicine and Research Institute of Clinical Medicine, Chonbuk National University Medical School and Hospital, Jeonju, Korea.
- 12Department of Infectious Disease, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2391426
- DOI: http://doi.org/10.3947/ic.2017.49.3.205
Abstract
- BACKGROUND
Abacavir is a widely-used nucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus (HIV) infection. Mandatory postmarketing surveillance was conducted in Korea to monitor the safety and evaluate the effectiveness of Ziagen® (abacavir sulfate 300 mg; ViiV Healthcare, Middlesex, UK).
MATERIALS AND METHODS
An open-label, multi-center, non-interventional postmarketing surveillance study was conducted from June 2010 to June 2016 to monitor the safety and effectiveness of Ziagen across 12 hospitals in Korea. Subjects older than 18 years taking Ziagen according to prescribing information were enrolled. The primary outcome was defined as the occurrence of any adverse events after Ziagen administration. Secondary outcomes included the occurrence of adverse drug reactions, occurrence of serious adverse events, and effectiveness of Ziagen administration.
RESULTS
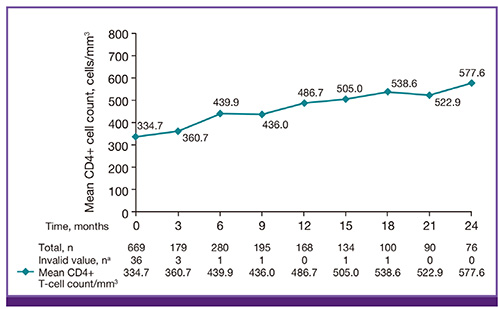
A total of 669 patients were enrolled in this study, with a total observation period of 1047.8 person-years. Of these, 90.7% of patients were male. The mean age of patients was 45.8±11.9 years. One-hundred ninety-six (29.3%) patients reported 315 adverse events, and four patients reported seven serious adverse events, without any fatal events. There was one potential case of an abacavir hypersensitivity reaction. Among the 97 adverse drug reactions that were reported from 75 patients, the most frequent adverse drug reactions included diarrhea (12 events), dyspepsia (10 events), and rash (9 events). No ischemic heart disease was observed. In the effectiveness analysis, 91% of patients achieved HIV-1 RNA under 50 copies/mL after 24 months of observation with abacavir administration.
CONCLUSION
Our data showed the safety and effectiveness of Ziagen in a real-world setting. During the study period, Ziagen was well-tolerated, with one incident of a clinically suspected abacavir hypersensitivity reaction. The postmarketing surveillance of Ziagen did not highlight any new safety information. These data may be helpful in understanding abacavir and the HIV treatment practices in Korea.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Regional Experience of Abacavir: Valuable but Still has Unanswered Question
Ji Hwan Bang
Infect Chemother. 2017;49(3):241-242. doi: 10.3947/ic.2017.49.3.241.Safety and Effectiveness Analysis of Kivexa® (lamivudine/abacavir sulfate) in Human Immunodeficiency Virus Infected Korean Patients
Heawon Ann, Yil-Seob Lee, Yeon-Sook Kim, Sook-In Jung, Sun-Hee Lee, Chang-Seop Lee, Jin-Soo Lee, Won Suk Choi, Young Hwa Choi, Shin-Woo Kim
Infect Chemother. 2019;51(2):150-160. doi: 10.3947/ic.2019.51.2.150.
Reference
-
1. Staszewski S, Katlama C, Harrer T, Massip P, Yeni P, Cutrell A, Tortell SM, Harrigan RP, Steel H, Lanier RE, Pearce G. A dose-ranging study to evaluate the safety and efficacy of abacavir alone or in combination with zidovudine and lamivudine in antiretroviral treatment-naive subjects. AIDS. 1998; 12:F197–F202.
Article2. Staszewski S, Keiser P, Montaner J, Raffi F, Gathe J, Brotas V, Hicks C, Hammer SM, Cooper D, Johnson M, Tortell S, Cutrell A, Thorborn D, Isaacs R, Hetherington S, Steel H, Spreen W; CNAAB3005 International Study Team. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: A randomized equivalence trial. JAMA. 2001; 285:1155–1163.
Article3. Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F, Hocqueloux L, Maggiolo F, Sandkovsky U, Granier C, Pappa K, Wynne B, Min S, Nichols G. SINGLE Investigators. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013; 369:1807–1818.
Article4. DeJesus E, Herrera G, Teofilo E, Gerstoft J, Buendia CB, Brand JD, Brothers CH, Hernandez J, Castillo SA, Bonny T, Lanier ER, Scott TR; CNA30024 Study Team. Abacavir versus zidovudine combined with lamivudine and efavirenz, for the treatment of antiretroviral-naive HIV-infected adults. Clin Infect Dis. 2004; 39:1038–1046.
Article5. The Korean Society for AIDS. Clinical guidelines for the diagnosis and treatment of HIV/AIDS in HIV-infected Koreans. Infect Chemother. 2011; 43:89–128.6. AIDS info. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Accessed 14 June, 2017. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.7. Günthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, Hoy JF, Mugavero MJ, Sax PE, Thompson MA, Gandhi RT, Landovitz RJ, Smith DM, Jacobsen DM, Volberding PA. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society-USA panel. JAMA. 2016; 316:191–210.
Article8. Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jägel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A; PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008; 358:568–579.
Article9. Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011; 25:1289–1298.
Article10. Cruciani M, Zanichelli V, Serpelloni G, Bosco O, Malena M, Mazzi R, Mengoli C, Parisi SG, Moyle G. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS. 2011; 25:1993–2004.11. Ding X, Andraca-Carrera E, Cooper C, Miele P, Kornegay C, Soukup M, Marcus KA. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr. 2012; 61:441–447.12. Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, Obel N. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS. 2011; 25:1637–1646.13. The Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment. Accessed 14 June, 2017. Available at: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf.14. National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015; 66:884–930.15. Korean Society for AIDS. The 2015 clinical guidelines for the diagnosis and treatment of HIV/AIDS in HIV-infected Koreans. Infect Chemother. 2015; 47:205–211.16. Korea Centers for Disease Control and Prevention (KCDC). Annual report on the notified HIV/AIDS in Korea. Accessed 14 June, 2017. Available at: http://cdc.go.kr/CDC/cms/content/mobile/59/19359_view.html.17. Korea Centers for Disease Control and Prevention (KCDC). Annual report on the notified HIV/AIDS in Korea. Accessed 14 June, 2017. Available at: http://cdc.go.kr/CDC/info/CdcKrInfo0128.jsp?menuIds=HOME001-MNU1130-MNU1156-MNU1426-MNU1448&cid=28485.18. Boettiger DC, Kerr S, Ditangco R, Merati TP, Pham TT, Chaiwarith R, Kiertiburanakul S, Li CK, Kumarasamy N, Vonthanak S, Lee C, Van Kinh N, Pujari S, Wong WW, Kamarulzaman A, Zhang F, Yunihastuti E, Choi JY, Oka S, Ng OT, Kantipong P, Mustafa M, Ratanasuwan W, Sohn A, Law M. Trends in first-line antiretroviral therapy in Asia: results from the TREAT Asia HIV observational database. PLoS One. 2014; 9:e106525.
Article19. Kim MJ, Kim SW, Chang HH, Kim Y, Jin S, Jung H, Park JH, Kim S, Lee JM. Comparison of antiretroviral regimens: adverse effects and tolerability failure that cause regimen switching. Infect Chemother. 2015; 47:231–238.
Article20. Kurita T, Kitaichi T, Nagao T, Miura T, Kitazono Y. Safety analysis of Ziagen® (abacavir sulfate) in postmarketing surveillance in Japan. Pharmacoepidemiol Drug Saf. 2014; 23:361–371.
Article21. Bannister WP, Friis-Møller N, Mocroft A, Viard JP, van Lunzen J, Kirk O, Gargalianos P, Bánhegyi D, Chiesi A, Lundgren JD. EuroSIDA Study Group. Incidence of abacavir hypersensitivity reactions in euroSIDA. Antivir Ther. 2008; 13:687–696.22. Hetherington S, McGuirk S, Powell G, Cutrell A, Naderer O, Spreen B, Lafon S, Pearce G, Steel H. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther. 2001; 23:1603–1614.
Article23. Saag M, Balu R, Phillips E, Brachman P, Martorell C, Burman W, Stancil B, Mosteller M, Brothers C, Wannamaker P, Hughes A, Sutherland-Phillips D, Mallal S, Shaefer M; Study of Hypersensitivity to Abacavir and Pharmacogenetic Evaluation Study Team. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008; 46:1111–1118.
Article24. Young B, Squires K, Patel P, Dejesus E, Bellos N, Berger D, Sutherland-Phillips DH, Liao Q, Shaefer M, Wannamaker P. First large, multicenter, open-label study utilizing HLA-B*5701 screening for abacavir hypersensitivity in North America. AIDS. 2008; 22:1673–1675.
Article25. Rauch A, Nolan D, Martin A, McKinnon E, Almeida C, Mallal S. Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV cohort study. Clin Infect Dis. 2006; 43:99–102.
Article26. Sun HY, Hung CC, Lin PH, Chang SF, Yang CY, Chang SY, Chang SC. Incidence of abacavir hypersensitivity and its relationship with HLA-B*5701 in HIV-infected patients in Taiwan. J Antimicrob Chemother. 2007; 60:599–604.
Article27. Phillips EJ. Genetic screening to prevent abacavir hypersensitivity reaction: are we there yet? Clin Infect Dis. 2006; 43:103–105.
Article28. Park WB, Choe PG, Song KH, Lee S, Jang HC, Jeon JH, Park SW, Park MH, Oh MD, Choe KW. Should HLA-B*5701 screening be performed in every ethnic group before starting abacavir? Clin Infect Dis. 2009; 48:365–367.
Article29. Kurita T, Kitaichi T, Nagao T, Miura T, Kitazono Y. Safety analysis of Epzicom® (lamivudine/abacavir sulfate) in post-marketing surveillance in Japan. Pharmacoepidemiol Drug Saf. 2014; 23:372–381.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alopecia Areata Associated with Abacavir Therapy
- Prevalence and Risk Factors of Low Bone Mineral Density in Korean HIV-Infected Patients: Impact of Abacavir and Zidovudine
- A Korean Post-Marketing Study of Abacavir/Dolutegravir/Lamivudine in Patients with HIV-1
- Hypertension Risk with Abacavir Use among HIV-Infected Individuals: A Nationwide Cohort Study
- A Case of Hypersensitivity Reaction Induced by Abacavir in an AIDS Patient