Infect Chemother.
2014 Jun;46(2):103-105. 10.3947/ic.2014.46.2.103.
Alopecia Areata Associated with Abacavir Therapy
- Affiliations
-
- 1Division of Epidemic Intelligence Service, Korea Centers for Disease Control and Prevention, Osan, Korea.
- 2Division of Infectious Disease, Department of Internal Medicine, National Medical Center, Seoul, Korea. hyoungsshin@gmail.com
- KMID: 2284986
- DOI: http://doi.org/10.3947/ic.2014.46.2.103
Abstract
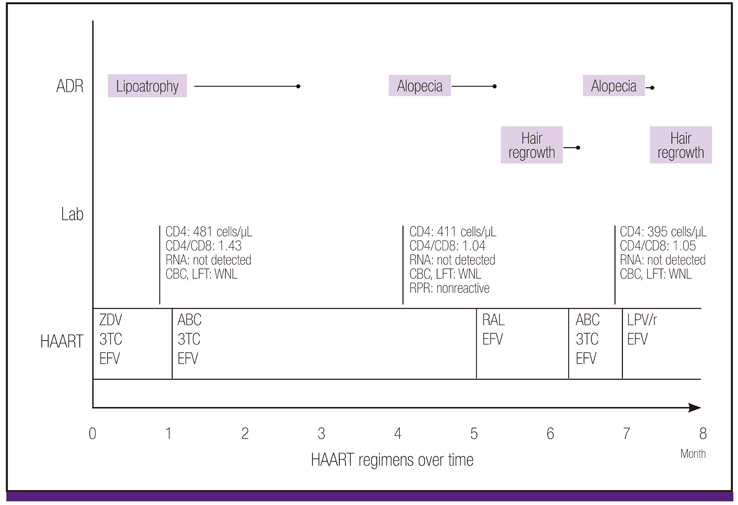
- Abacavir is a nucleoside reverse-transcriptase inhibitor that has been approved for use in combination with other retroviral agents in the treatment of HIV infection. Common adverse reactions include headache, fatigue, nausea, and rash. A fatal hypersensitivity reaction may occur in 5% of patients receiving abacavir; therefore, screening for HLA-B5701 should be performed before starting abacavir. Alopecia areata (AA) is infrequently reported in HIV-infected patients. Certain underlying conditions have been associated with AA, including a decreased CD4:CD8 ratio related to the progression of HIV infection, some opportunistic infections, and syphilis. Several antiretroviral drugs, such as zidovudine, indinavir, indinavir/ritonavir, lopinavir/ritonavir, and atazanavir/ritonavir have been implicated in the development of AA. At present, the occurrence of AA has not been associated with abacavir use. We cannot exclude that the use of abacavir and the development of AA could be coincidental. Nevertheless, patients given abacavir should be monitored for hair loss and the drug discontinued promptly if such signs appear.
Keyword
MeSH Terms
Figure
Reference
-
1. Tantikul C, Dhana N, Jongjarearnprasert K, Visitsunthorn N, Vichyanond P, Jirapongsananuruk O. The utility of the World Health Organization-The Uppsala Monitoring Centre (WHO-UMC) system for the assessment of adverse drug reactions in hospitalized children. Asian Pac J Allergy Immunol. 2008; 26:77–82.2. Lenhard JM, Welel JE, Paulik MA, Furfine ES. Stimulation of vitamin A(1) acid signaling by the HIV protease inhibitor indinavir. Biochem Pharmacol. 2000; 59:1063–1068.
Article3. Côté HC. Mechanisms of antiretroviral therapy-induced mitochondrial dysfunction. Curr Opin HIV AIDS. 2007; 2:253–260.
Article4. Virgili N, Fisac C, Martínez E, Ribera E, Gatell JM, Podzamczer D. Proinflammatory cytokine changes in clinically stable, virologically suppressed, HIV-infected patients switching from protease inhibitors to abacavir. J Acquir Immune Defic Syndr. 2009; 50:552–553.
Article5. Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004; 429:417–423.
Article6. Gilhar A, Kalish RS. Alopecia areata: a tissue specific autoimmune disease of the hair follicle. Autoimmun Rev. 2006; 5:64–69.
Article7. Sadick NS. Clinical and laboratory evaluation of AIDS trichopathy. Int J Dermatol. 1993; 32:33–38.
Article8. Jordaan HF, Louw M. The moth-eaten alopecia of secondary syphilis. A histopathological study of 12 patients. Am J Dermatopathol. 1995; 17:158–162.
Article9. Geletko SM, Segarra M, Mikolich DJ. Alopecia associated with zidovudine therapy. Pharmacotherapy. 1996; 16:79–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Congenitally Developed Alopecia Areata
- A Case of Alopecia Areata in a Patient taking Cyclosporine
- Superficial Cryotherapy of Alopecia Areata in Eyebrows
- Concurrent Appearance of Alopecia Areata in Four Siblings
- Efficacy of Oral Tofacitinib in Patients with Alopecia Areata: Tofacitinib Was Ineffective for Recalcitrant Alopecia Totalis of a Long Duration