Allergy Asthma Immunol Res.
2016 May;8(3):264-275. 10.4168/aair.2016.8.3.264.
CpG Oligodeoxynucleotide Inhibits Cockroach-Induced Asthma via Induction of IFN-gamma+ Th1 Cells or Foxp3+ Regulatory T Cells in the Lung
- Affiliations
-
- 1Department of Life Science, College of Natural Sciences, Hanyang University, Seoul, Korea. jeminchoi@hanyang.ac.kr
- 2Research Institute for Natural Sciences, Hanyang University, Seoul, Korea.
- 3Division of Allergy and Immunology, Department of Internal Medicine and Institute of Allergy, Yonsei University College of Medicine, Seoul, Korea. parkjw@yuhs.ac
- KMID: 2391047
- DOI: http://doi.org/10.4168/aair.2016.8.3.264
Abstract
- PURPOSE
CpG oligodeoxynucleotide (CpG-ODN), a TLR9 agonist, activates innate immunity and induces Th1 response. Although the immune modulatory effect of CpG-ODN has been extensively studied, its function in cockroach extract-induced allergic asthma has not been studied. Here, we investigated the inhibitory function of CpG-ODN in cockroach extract-induced asthma in mice with different treatment schemes.
METHODS
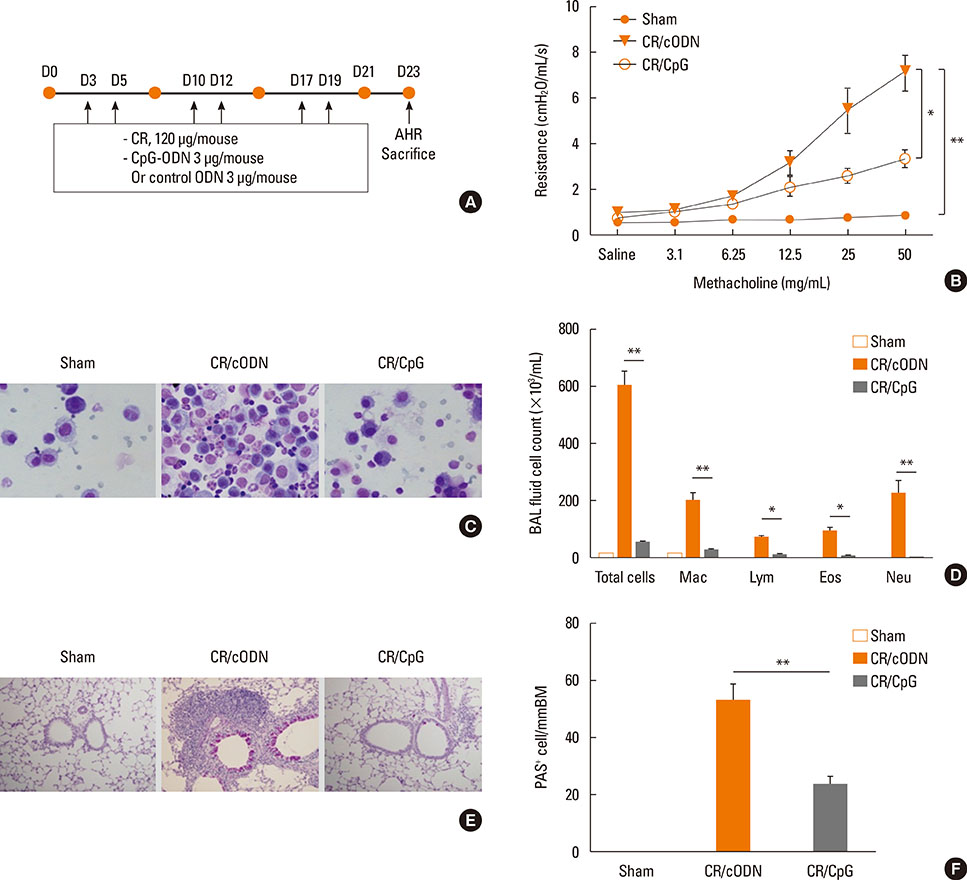
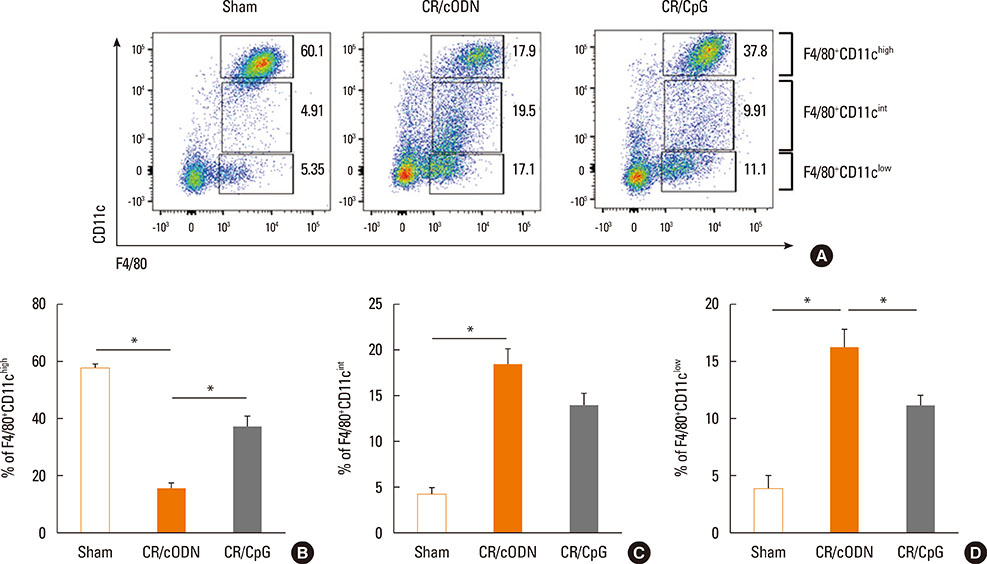
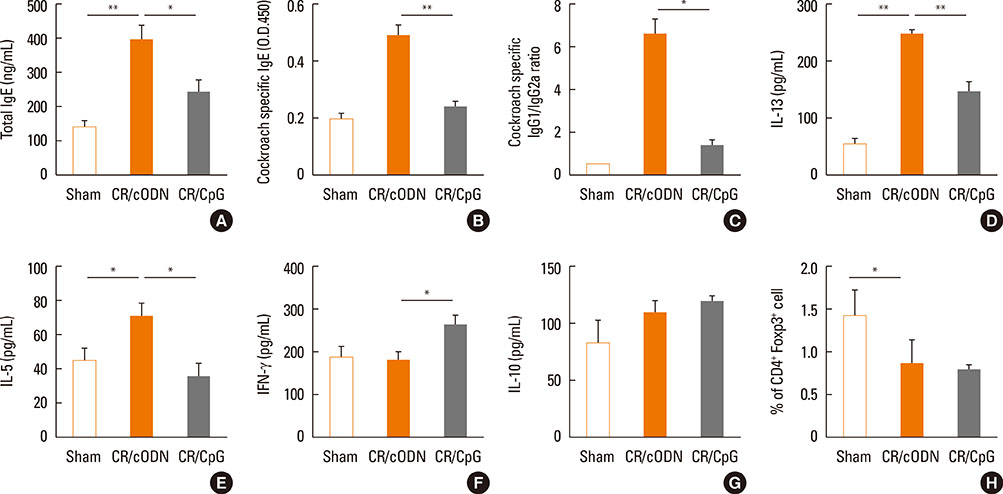
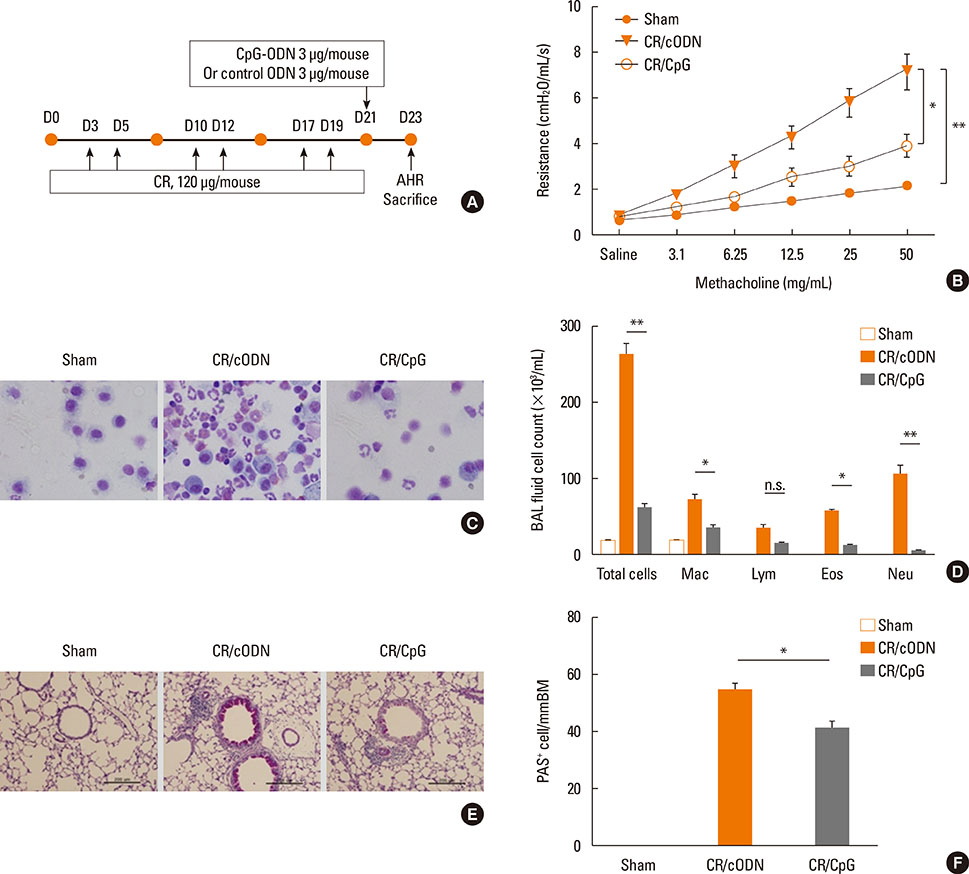
Scheme 1: BALB/C mice were intra-nasally co-administered by cockroach extract and CpG-ODN twice a week for 3 weeks; Scheme 2: The mice were intra-nasally pre-treated with CpG-ODN at day 0 and cockroach allergen challenge was performed from day 3 as in scheme 1. Scheme 3: Cockroach allergen challenge was performed as in scheme 1 and CpG-ODN was post-treated at day 21. Then, BAL cell count, flow cytometric analysis of alveolar macrophages, regulatory T cells, and lung tissue histology, Th1 and Th2 cytokines, serum IgE, cockroach specific IgE, IgG1/IgG2a ratio, and airway hyper-responsiveness were evaluated.
RESULTS
Mice with repeated intra-nasal exposure to CpG-ODN showed a dramatic decrease in eosinophilic inflammation, goblet cell hyperplasia, and airway hyper-responsiveness with reduction of IL-13, IL-5, and serum IgE, cockroach specific IgE and IgG1/IgG2a ratio. This inhibitory function might be related to the up-regulation of IL-10 and CD4+Foxp3+ regulatory T cells in the lung. Interestingly, one-time challenge of CpG-ODN either prior or posterior to cockroach extract exposure could modulate airway inflammation and hyper-responsiveness via increase of Th1 response.
CONCLUSIONS
Collectively, our data suggest that CpG-ODN treatment modulates Th2 inflammation in the lung by induction of regulatory T cells or Th1 response in a cockroach-induced asthma model.
MeSH Terms
-
Animals
Asthma*
Cell Count
Cockroaches
Cytokines
Eosinophils
Goblet Cells
Hyperplasia
Immunity, Innate
Immunoglobulin E
Inflammation
Interleukin-10
Interleukin-13
Interleukin-5
Lung*
Macrophages, Alveolar
Mice
T-Lymphocytes, Regulatory*
Th1 Cells*
Up-Regulation
Cytokines
Immunoglobulin E
Interleukin-10
Interleukin-13
Interleukin-5
Figure
Reference
-
1. Djukanović R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, et al. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990; 142:434–457.2. Jeong KY, Son M, Lee JH, Hong CS, Park JW. Allergenic characterization of a novel allergen, homologous to chymotrypsin, from german cockroach. Allergy Asthma Immunol Res. 2015; 7:283–289.3. Park HJ, Lee JH, Park KH, Ann HW, Jin MN, Choi SY, et al. A nationwide survey of inhalant allergens sensitization and levels of indoor major allergens in Korea. Allergy Asthma Immunol Res. 2014; 6:222–227.4. Robinson D, Hamid Q, Bentley A, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993; 92:313–324.5. Ray A, Cohn L. Altering the Th1/Th2 balance as a therapeutic strategy in asthmatic diseases. Curr Opin Investig Drugs. 2000; 1:442–448.6. Barnes PJ. New drugs for asthma. Discov Med. 2004; 4:421–426.7. Krieg AM, Matson S, Fisher E. Oligodeoxynucleotide modifications determine the magnitude of B cell stimulation by CpG motifs. Antisense Nucleic Acid Drug Dev. 1996; 6:133–139.8. Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995; 374:546–549.9. Schwartz DA, Quinn TJ, Thorne PS, Sayeed S, Yi AK, Krieg AM. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest. 1997; 100:68–73.10. Chace JH, Hooker NA, Mildenstein KL, Krieg AM, Cowdery JS. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol. 1997; 84:185–193.11. Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996; 93:2879–2883.12. Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997; 186:1623–1631.13. Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Weinstock JV, Thorne PS, et al. Modulation of airway inflammation by CpG oligo-deoxynucleotides in a murine model of asthma. J Immunol. 1998; 160:2555–2559.14. Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001; 31:2154–2163.15. Takauji R, Iho S, Takatsuka H, Yamamoto S, Takahashi T, Kitagawa H, et al. CpG-DNA-induced IFN-alpha production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J Leukoc Biol. 2002; 72:1011–1019.16. Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004; 173:4433–4442.17. Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009; 183:2475–2483.18. Kline JN, Ballas ZK. DNA immunomodulation of asthma. Clin Allergy Immunol. 2002; 16:551–564.19. Krieg AM, Kline JN. Immune effects and therapeutic applications of CpG motifs in bacterial DNA. Immunopharmacology. 2000; 48:303–305.20. Mendoza J, Snyder RD. Cockroach sensitivity in children with bronchial asthma. Ann Allergy. 1970; 28:159–163.21. Kang B. Study on cockroach antigen as a probable causative agent in bronchial asthma. J Allergy Clin Immunol. 1976; 58:357–365.22. Huss K, Adkinson NF Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001; 107:48–54.23. Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J Allergy Clin Immunol. 2001; 107:41–47.24. Stelmach I, Brzozowska A, Jerzyńska J, Podsiadłowicz-Borzecka M, Bobrowska M, Majak P, et al. Hypersensitivity to cockroach allergen among children with bronchial asthma living in the Łódź district. Pol Merkur Lekarski. 2000; 9:657–661.25. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997; 336:1356–1363.26. Schulaner FA. Cockroach allergen and asthma. N Engl J Med. 1997; 337:791–792.27. Kline JN, Kitagaki K, Businga TR, Jain VV. Treatment of established asthma in a murine model using CpG oligodeoxynucleotides. Am J Physiol Lung Cell Mol Physiol. 2002; 283:L170–L179.28. Hirose I, Tanaka H, Takahashi G, Wakahara K, Tamari M, Sakamoto T, et al. Immunomodulatory effects of CpG oligodeoxynucleotides on house dust mite-induced airway inflammation in mice. Int Arch Allergy Immunol. 2008; 147:6–16.29. Jeong KY, Kim CR, Park J, Han IS, Park JW, Yong TS. Identification of novel allergenic components from German cockroach fecal extract by a proteomic approach. Int Arch Allergy Immunol. 2013; 161:315–324.30. Park JW, Taube C, Joetham A, Takeda K, Kodama T, Dakhama A, et al. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am J Respir Crit Care Med. 2004; 169:726–732.31. Mathias LJ, Khong SM, Spyroglou L, Payne NL, Siatskas C, Thorburn AN, et al. Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. J Immunol. 2013; 191:5914–5924.32. Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001; 70:163–170.33. Arnoldussen DL, Linehan M, Sheikh A. BCG vaccination and allergy: a systematic review and meta-analysisl. J Allergy Clin Immunol. 2011; 127:246–253. 253.e1–253.e21.34. Kim YJ, Kim HJ, Kang MJ, Yu HS, Seo JH, Kim HY, et al. Bacillus Calmette-Guérin Suppresses Asthmatic Responses via CD4(+)CD25(+) Regulatory T Cells and Dendritic Cells. Allergy Asthma Immunol Res. 2014; 6:201–207.35. Warren TL, Bhatia SK, Acosta AM, Dahle CE, Ratliff TL, Krieg AM, et al. APC stimulated by CpG oligodeoxynucleotide enhance activation of MHC class I-restricted T cells. J Immunol. 2000; 165:6244–6251.36. Tokunaga T, Yamamoto H, Shimada S, Abe H, Fukuda T, Fujisawa Y, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984; 72:955–962.37. Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T, et al. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol. 1992; 36:983–997.38. Krug A, Rothenfusser S, Selinger S, Bock C, Kerkmann M, Battiany J, et al. CpG-A oligonucleotides induce a monocyte-derived dendritic cell-like phenotype that preferentially activates CD8 T cells. J Immunol. 2003; 170:3468–3477.39. Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003; 198:513–520.40. Sur S, Wild JS, Choudhury BK, Sur N, Alam R, Klinman DM. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol. 1999; 162:6284–6293.41. Kline JN, Krieg AM, Waldschmidt TJ, Ballas ZK, Jain V, Businga TR. CpG oligodeoxynucleotides do not require TH1 cytokines to prevent eosinophilic airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 1999; 104:1258–1264.42. Baban B, Chandler PR, Johnson BA 3rd, Huang L, Li M, Sharpe ML, et al. Physiologic control of IDO competence in splenic dendritic cells. J Immunol. 2011; 187:2329–2335.43. Volpi C, Fallarino F, Pallotta MT, Bianchi R, Vacca C, Belladonna ML, et al. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat Commun. 2013; 4:1852.44. Teleshova N, Kenney J, Jones J, Marshall J, Van Nest G, Dufour J, et al. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN-gamma-secreting simian immunodeficiency virus-specific T cells. J Immunol. 2004; 173:1647–1657.45. Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol. 2002; 168:1659–1663.46. Klinman DM, Xie H, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the licensed anthrax vaccine. Ann N Y Acad Sci. 2006; 1082:137–150.47. Weigel BJ, Rodeberg DA, Krieg AM, Blazar BR. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin Cancer Res. 2003; 9:3105–3114.48. Mousavi T, Salek Moghadam A, Falak R, Tebyanian M. Co-administration of CpG oligonucleotides and chenopodium album extract reverse IgG2a/IgG1 ratios and increase IFN-gamma and IL-10 productions in a murine model of asthma. Iran J Allergy Asthma Immunol. 2008; 7:1–6.49. Kitagaki K, Jain VV, Businga TR, Hussain I, Kline JN. Immunomodulatory effects of CpG oligodeoxynucleotides on established th2 responses. Clin Diagn Lab Immunol. 2002; 9:1260–1269.50. Mason KA, Hunter NR. CpG plus radiotherapy: a review of preclinical works leading to clinical trial. Front Oncol. 2012; 2:101.51. Zent CS, Smith BJ, Ballas ZK, Wooldridge JE, Link BK, Call TG, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma. 2012; 53:211–217.52. Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligode-oxynucleotides as immune adjuvants. Immunol Rev. 2004; 199:201–216.53. Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008; 27:161–167.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intranasal Administration of Unmethylated CpG with Cockroach Antigen Prevents the Development of Cockroach-Induced Allergic Inflammation
- Adenovirus-mediated Foxp3 expression in lung epithelial cells reduces airway inflammation in ovalbumin and cockroach-induced asthma model
- TLR4, 5, and 9 Agonists Inhibit Murine Airway Invariant Natural Killer T Cells in an IL-12-Dependent Manner
- In Vitro Induction of Allergen-Specific Interleukin-10-Producing Regulatory B Cell Responses by Interferon-gamma in Non-Immunoglobulin E-Mediated Milk Allergy
- Anti-proliferative Activity of T-bet