Allergy Asthma Immunol Res.
2012 Sep;4(5):295-304. 10.4168/aair.2012.4.5.295.
TLR4, 5, and 9 Agonists Inhibit Murine Airway Invariant Natural Killer T Cells in an IL-12-Dependent Manner
- Affiliations
-
- 1Department of Allergy, Asthma and Clinical Immunology, Chonnam National University Medical School, Gwangju, Korea. yikoh@chonnam.ac.kr
- 2Department of Microbiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2167049
- DOI: http://doi.org/10.4168/aair.2012.4.5.295
Abstract
- PURPOSE
Invariant natural killer T (iNKT) cells may play an important role in the pathogenesis of asthma in mice and humans. Thus, an agent that modulates the function of iNKT cells may have therapeutic potential to control asthma. We hypothesized that lipopolysaccharide (LPS)-, flagellin-, or CpG-induced changes in the cytokine milieu may modify and even inhibit the function of airway iNKT cells in asthma.
METHODS
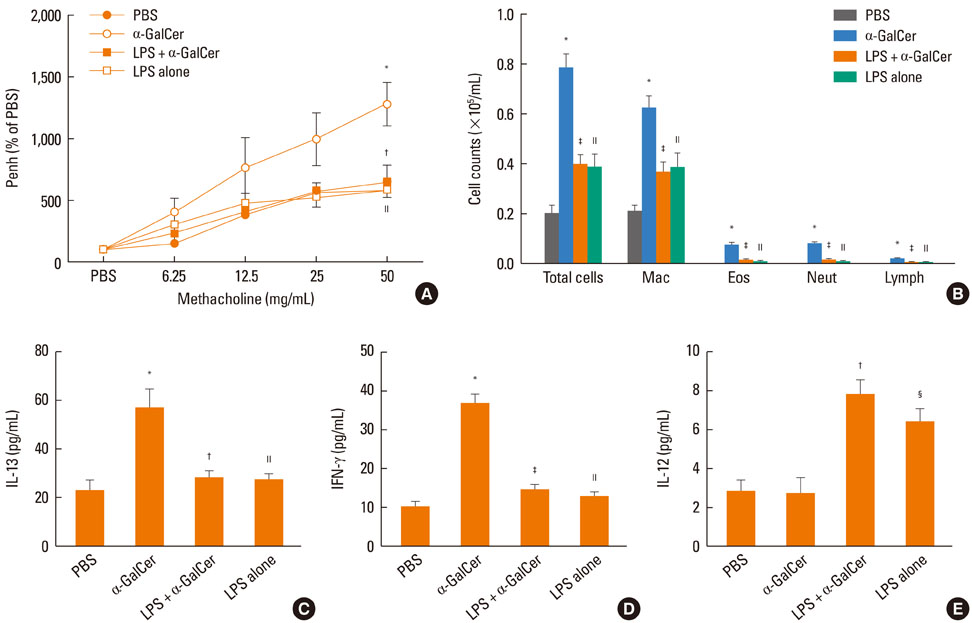
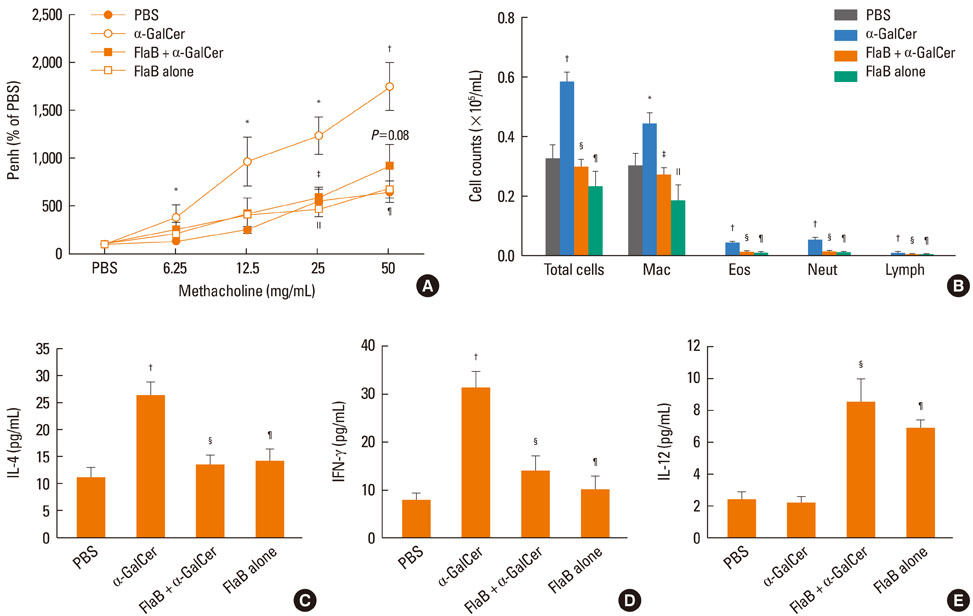
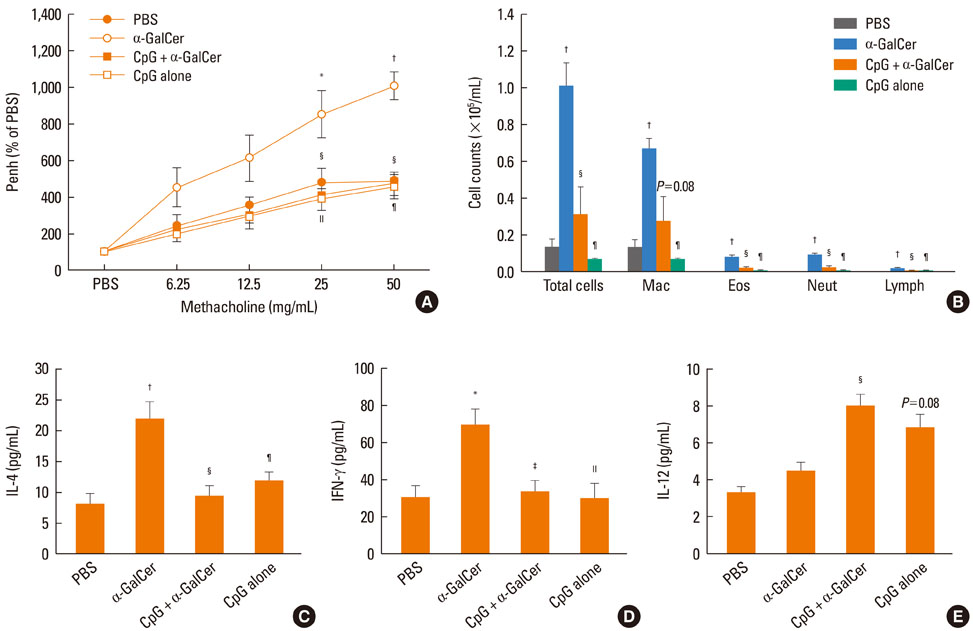
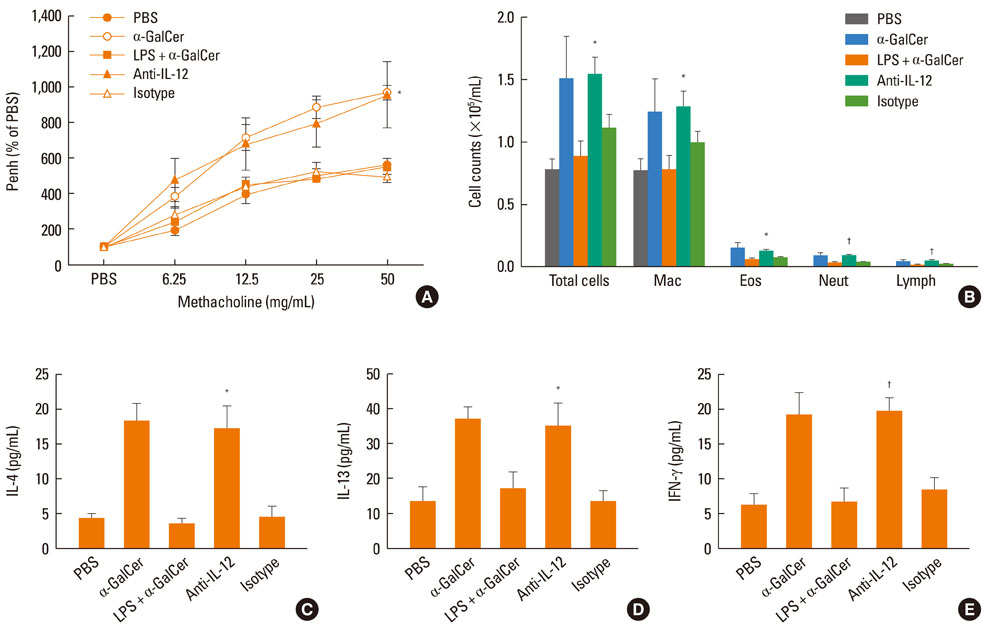
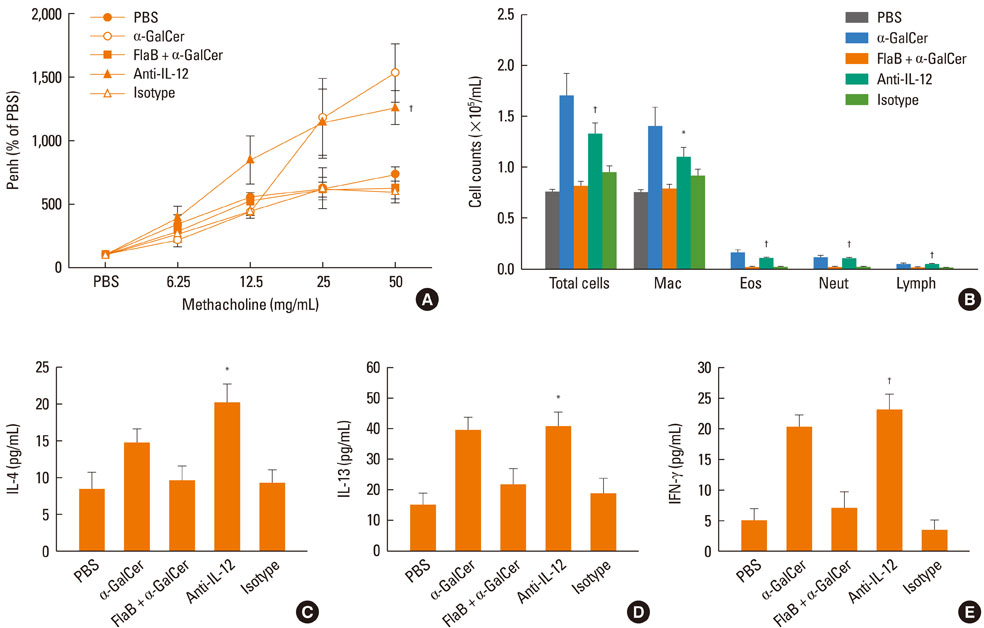
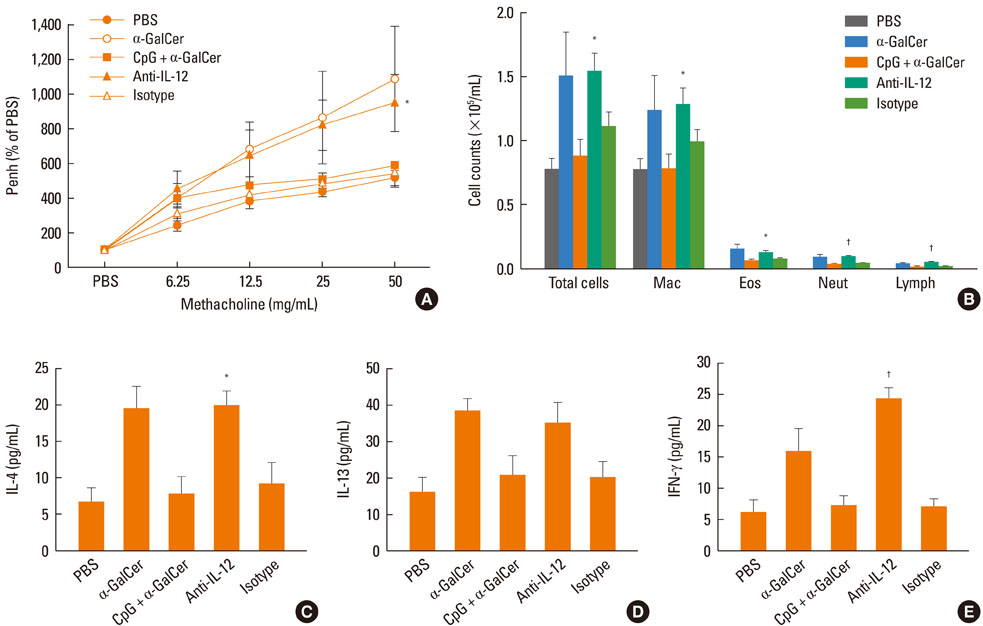
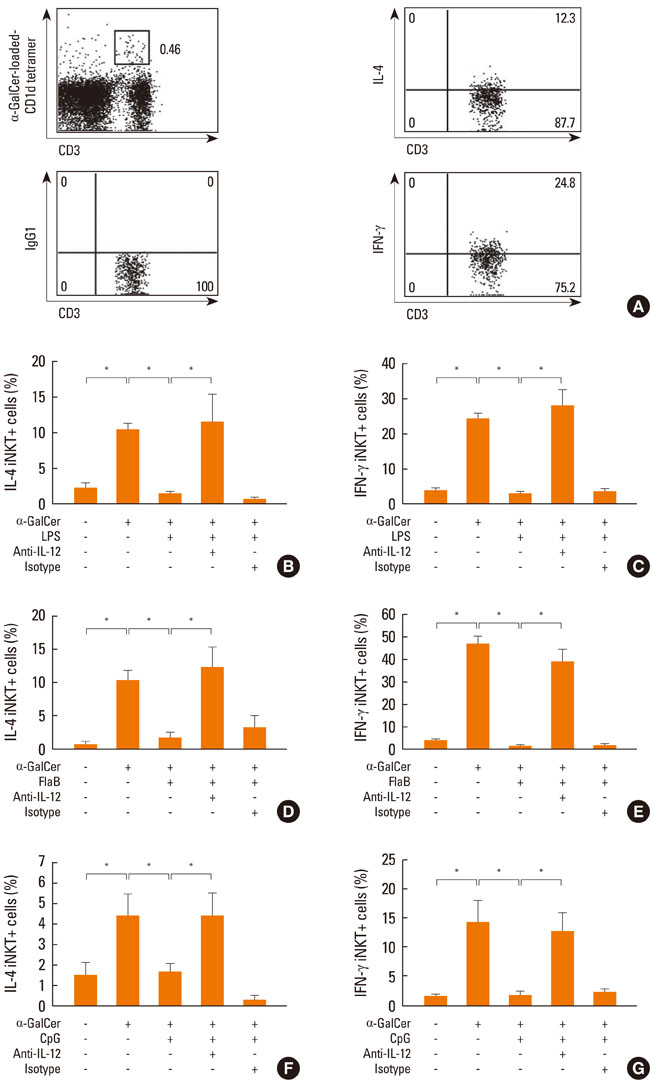
Because increased alpha-galactosylceramide (GalCer)-induced airway hyperreactivity (AHR) reflects the presence of airway iNKT cells, alpha-GalCer-induced AHR, as well as inflammatory cells and cytokines in bronchoalveolar lavage (BAL) fluid, were determined 24 hours after in vivo treatment with LPS, flagellin, or CpG in naive BALB/c mice. Intracellular IL-4 and IFN-gamma were measured in spleen iNKT cells after in vitro treatment with LPS, flagellin, or CpG. A role for IL-12 following the treatments was determined.
RESULTS
Intranasal administration of LPS, flagellin, or CpG reduced development of alpha-GalCer-induced AHR, eosinophilic airway inflammation, and Th1 and Th2 cytokine responses in BAL fluid, while producing IL-12 in BAL fluid. Intraperitoneal administration of IL-12 mAb blocked the suppressive effect of LPS, flagellin, or CpG. In vitro treatment with LPS, flagellin, or CpG reduced production of IL-4 and IFN-gamma from alpha-GalCer-stimulated spleen iNKT cells; these effects were ameliorated by addition of anti-IL-12 mAb.
CONCLUSIONS
TLR4, 5, and 9 agonists may suppress the function of airway and spleen iNKT cells via IL-12-dependent mechanisms. Anergy of iNKT cells by IL-12 might play a role in suppression by these TLR agonists.
Keyword
MeSH Terms
Figure
Reference
-
1. Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002. 2:557–568.2. Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003. 9:582–588.3. Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci U S A. 2006. 103:2782–2787.4. Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008. 205:385–393.5. Koh YI, Kim HY, Meyer EH, Pichavant M, Akbari O, Yasumi T, Savage PB, Dekruyff RH, Umetsu DT. Activation of nonclassical CD1d-restricted NK T cells induces airway hyperreactivity in beta 2-microglobulin-deficient mice. J Immunol. 2008. 181:4560–4569.6. Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlström J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006. 354:1117–1129.7. Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, Gadola SD, Friedmann PS, Djukanovic R. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N Engl J Med. 2007. 356:1410–1422.8. Koh YI, Shim JU, Wi JO, Han ER, Jin NC, Oh SH, Park CK, Park DJ. Inverse association of peripheral blood CD4(+) invariant natural killer T cells with atopy in human asthma. Hum Immunol. 2010. 71:186–191.9. Koh YI, Shim JU. Association between sputum natural killer T cells and eosinophilic airway inflammation in human asthma. Int Arch Allergy Immunol. 2010. 153:239–248.10. Koh YI, Shim JU, Wi J, Kwon YE. The Role of Natural Killer T Cells in the Pathogenesis of Acute Exacerbation of Human Asthma. Int Arch Allergy Immunol. 2012. 158:131–141.11. Matsuda H, Suda T, Sato J, Nagata T, Koide Y, Chida K, Nakamura H. alpha-Galactosylceramide, a ligand of natural killer T cells, inhibits allergic airway inflammation. Am J Respir Cell Mol Biol. 2005. 33:22–31.12. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006. 355:2226–2235.13. Nauta AJ, Engels F, Knippels LM, Garssen J, Nijkamp FP, Redegeld FA. Mechanisms of allergy and asthma. Eur J Pharmacol. 2008. 585:354–360.14. Matricardi PM, Bjorksten B, Bonini S, Bousquet J, Djukanovic R, Dreborg S, Gereda J, Malling HJ, Popov T, Raz E, Renz H, Wold A. EAACI Task Force 7. Microbial products in allergy prevention and therapy. Allergy. 2003. 58:461–471.15. Bortolatto J, Borducchi E, Rodriguez D, Keller AC, Faquim-Mauro E, Bortoluci KR, Mucida D, Gomes E, Christ A, Schnyder-Candrian S, Schnyder B, Ryffel B, Russo M. Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: role of MyD88 adaptor molecule and interleukin-12/interferon-gamma axis. Clin Exp Allergy. 2008. 38:1668–1679.16. Lee SE, Koh YI, Kim MK, Kim YR, Kim SY, Nam JH, Choi YD, Bae SJ, Ko YJ, Ryu HJ, Koh JT, Choy HE, Rhee JH. Inhibition of airway allergic disease by co-administration of flagellin with allergen. J Clin Immunol. 2008. 28:157–165.17. Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, Van Uden J, Martin-Orozco E, Gelfand EW, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998. 161:7054–7062.18. Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007. 178:2706–2713.19. Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J Clin Invest. 2008. 118:2301–2315.20. Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008. 181:4452–4456.21. Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol. 2004. 16:241–247.22. Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005. 115:2572–2583.23. Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005. 175:3092–3101.24. Koh YI, Shim JU, Lee JH, Chung IJ, Min JJ, Rhee JH, Lee HC, Chung DH, Wi JO. Natural killer T cells are dispensable in the development of allergen-induced airway hyperresponsiveness, inflammation and remodelling in a mouse model of chronic asthma. Clin Exp Immunol. 2010. 161:159–170.25. Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galán JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006. 103:12487–12492.26. Paget C, Bialecki E, Fontaine J, Vendeville C, Mallevaey T, Faveeuw C, Trottein F. Role of invariant NK T lymphocytes in immune responses to CpG oligodeoxynucleotides. J Immunol. 2009. 182:1846–1853.27. Schwartz RH. T cell anergy. Annu Rev Immunol. 2003. 21:305–334.28. Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007. 27:597–609.29. Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003. 4:1230–1237.30. Kim DH, Chang WS, Lee YS, Lee KA, Kim YK, Kwon BS, Kang CY. 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol. 2008. 180:2062–2068.31. Grela F, Aumeunier A, Bardel E, Van LP, Bourgeois E, Vanoirbeek J, Leite-de-Moraes M, Schneider E, Dy M, Herbelin A, Thieblemont N. The TLR7 agonist R848 alleviates allergic inflammation by targeting invariant NKT cells to produce IFN-gamma. J Immunol. 2011. 186:284–290.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Th2-like Invariant Natural Killer T cells in Peripheral Blood From Patients With Asthma
- Flagellin Modulates the Function of Invariant NKT Cells From Patients With Asthma via Dendritic Cells
- Lineage Differentiation Program of Invariant Natural Killer T Cells
- Comparison of Invariant NKT Cells with Conventional T Cells by Using Gene Set Enrichment Analysis (GSEA)
- Out-sourcing for Trans-presentation: Assessing T Cell Intrinsic and Extrinsic IL-15 Expression with Il15 Gene Reporter Mice