Immune Netw.
2015 Aug;15(4):199-205. 10.4110/in.2015.15.4.199.
Anti-proliferative Activity of T-bet
- Affiliations
-
- 1College of Pharmacy and Graduate School of Pharmaceutical Sciences, Ewha Womans University, Seoul 03760, Korea. eshwang@ewha.ac.kr
- KMID: 2168047
- DOI: http://doi.org/10.4110/in.2015.15.4.199
Abstract
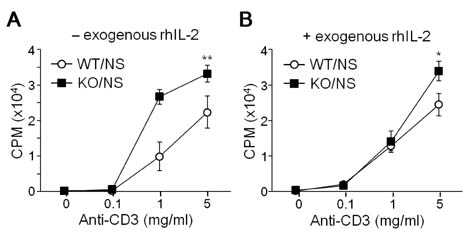
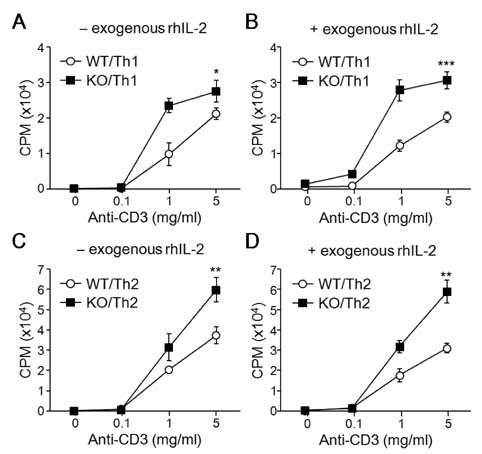
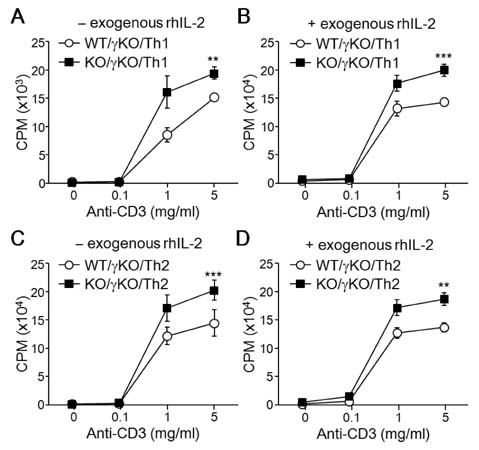
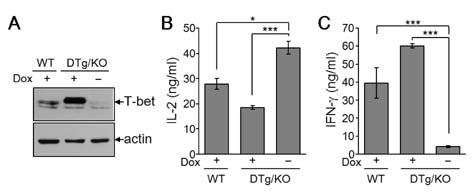
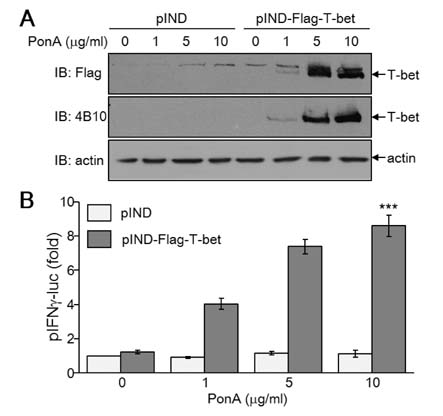
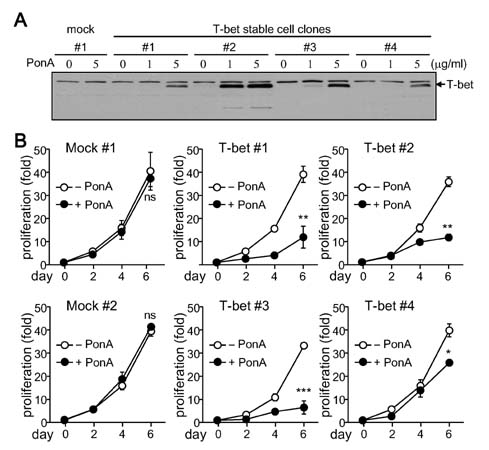
- T-bet is a critical transcription factor that regulates differentiation of Th1 cells from CD4+ precursor cells. Since T-bet directly binds to the promoter of the IFN-gamma gene and activates its transcription, T-bet deficiency impairs IFN-gamma production in Th1 cells. Interestingly, T-bet-deficient Th cells also display substantially augmented the production of IL-2, a T cell growth factor. Exogenous expression of T-bet in T-bet deficient Th cells rescued the IFN-gamma production and suppressed IL-2 expression. IFN-gamma and IL-2 reciprocally regulate Th cell proliferation following TCR stimulation. Therefore, we examined the effect of T-bet on Th cell proliferation and found that T-bet deficiency significantly enhanced Th cell proliferation under non-skewing, Th1-skewing, and Th2-skewing conditions. By using IFN-gamma-null mice to eliminate the anti-proliferative effect of IFN-gamma, T-bet deficiency still enhanced Th cell proliferation under both Th1- and Th2-skewing conditions. Since the anti-proliferative activity of T-bet may be influenced by IL-2 suppression in Th cells, we examined whether T-bet modulates IL-2-independent cell proliferation in a non-T cell population. We demonstrated that T-bet expression induced by ecdysone treatment in human embryonic kidney (HEK) cells increased IFN-gamma promoter activity in a dose dependent manner, and sustained T-bet expression considerably decreased cell proliferation in HEK cells. Although the molecular mechanisms underlying anti-proliferative activity of T-bet remain to be elucidated, T-bet may directly suppress cell proliferation in an IFN-gamma- or an IL-2-independent manner.
MeSH Terms
Figure
Reference
-
1. Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013; 13:777–789.
Article2. Hoyler T, Connor CA, Kiss EA, Diefenbach A. T-bet and Gata3 in controlling type 1 and type 2 immunity mediated by innate lymphoid cells. Curr Opin Immunol. 2013; 25:139–147.
Article3. Miller SA, Weinmann AS. Molecular mechanisms by which T-bet regulates T-helper cell commitment. Immunol Rev. 2010; 238:233–246.
Article4. Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing Y, Deng Z, Yao Z, Tsun A, Li B. FOXP3 and RORgammat: transcriptional regulation of Treg and Th17. Int Immunopharmacol. 2011; 11:536–542.5. Ziegler SF, Buckner JH. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect. 2009; 11:594–598.
Article6. Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011; 23:598–604.
Article7. Lan RY, Selmi C, Gershwin ME. The regulatory, inflammatory, and T cell programming roles of interleukin-2 (IL-2). J Autoimmun. 2008; 31:7–12.
Article8. Letourneau S, Krieg C, Pantaleo G, Boyman O. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J Allergy Clin Immunol. 2009; 123:758–762.
Article9. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000; 100:655–669.
Article10. Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O'Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001; 98:15137–15142.11. Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001; 292:1907–1910.
Article12. Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, Drazen JM, De Sanctis GT, Glimcher LH. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002; 295:336–338.
Article13. Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002; 295:338–342.
Article14. Werneck MB, Lugo-Villarino G, Hwang ES, Cantor H, Glimcher LH. T-bet plays a key role in NK-mediated control of melanoma metastatic disease. J Immunol. 2008; 180:8004–8010.
Article15. Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005; 307:430–433.
Article16. Park JW, Min HJ, Sohn JH, Kim JY, Hong JH, Sigrist KS, Glimcher LH, Hwang ES. Restoration of T-box-containing protein expressed in T cells protects against allergen-induced asthma. J Allergy Clin Immunol. 2009; 123:479–485.
Article17. Lee K, Min HJ, Jang EJ, Hong JH, Hwang ES. In vivo tumor suppression activity by T cell-specific T-bet restoration. Int J Cancer. 2010; 127:2129–2137.
Article18. Hwang ES, Hong JH, Glimcher LH. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J Exp Med. 2005; 202:1289–1300.
Article19. Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011; 12:96–104.
Article20. Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity. 2012; 37:501–510.
Article21. McPherson RC, Turner DG, Mair I, O'Connor RA, Anderton SM. T-bet Expression by Foxp3(+) T Regulatory Cells is Not Essential for Their Suppressive Function in CNS Autoimmune Disease or Colitis. Front Immunol. 2015; 6:69.
Article22. Hwang ES. Transcriptional regulation of T helper 17 cell differentiation. Yonsei Med J. 2010; 51:484–491.
Article23. Jang EJ, Park HR, Hong JH, Hwang ES. Lysine 313 of T-box is crucial for modulation of protein stability, DNA binding, and threonine phosphorylation of T-bet. J Immunol. 2013; 190:5764–5770.
Article24. Oh S, Hwang ES. The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells. J Immunol Res. 2014; 2014:589672.
Article25. Zamorano J, Wang HY, Wang R, Shi Y, Longmore GD, Keegan AD. Regulation of cell growth by IL-2: role of STAT5 in protection from apoptosis but not in cell cycle progression. J Immunol. 1998; 160:3502–3512.26. Ben-Sasson SZ, Le GG, Conrad DH, Finkelman FD, Paul WE. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990; 145:1127–1136.27. Hwang ES, White IA, Ho IC. An IL-4-independent and CD25-mediated function of c-maf in promoting the production of Th2 cytokines. Proc Natl Acad Sci U S A. 2002; 99:13026–13030.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Balloon Expulsion Test Does Not Seem to Be Useful for Screening or Exclusion of Dyssynergic Defecation as a Single Test
- Cellular Sources of T-bet in Asthma are Determined by Atopic Status
- Relationship between Proliferative Activity and Expression of HBcAg and p53 Protein in Hepatocellular Carcinoma and Surrounding Nontumorous Liver
- Telomerase Activity of Endometrium Related to the Effects of the Sex Steroid Hormone and Endometrial Cancer
- Mucosal Immunity Related to CD8+ T Lymphocytes in Children with Helicobacter pylori Gastritis