Cancer Res Treat.
2017 Jul;49(3):643-655. 10.4143/crt.2016.168.
Antitumor Effect of KX-01 through Inhibiting Src Family Kinases and Mitosis
- Affiliations
-
- 1Cancer Research Institute, Seoul National University, Seoul, Korea. moisa@snu.ac.kr
- 2Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 5Kinex Pharmaceutical Corporation, New York State Center of Excellence in Bioinformartics and Life Sciences, NY, USA.
- KMID: 2388310
- DOI: http://doi.org/10.4143/crt.2016.168
Abstract
- PURPOSE
KX-01 is a novel dual inhibitor of Src and tubulin. Unlike previous Src inhibitors that failed to show clinical benefit during treatment of breast cancer, KX-01 can potentially overcome the therapeutic limitations of current Src inhibitors through inhibition of both Src and tubulin. The present study further evaluates the activity and mechanism of KX-01 in vitro and in vivo.
MATERIALS AND METHODS
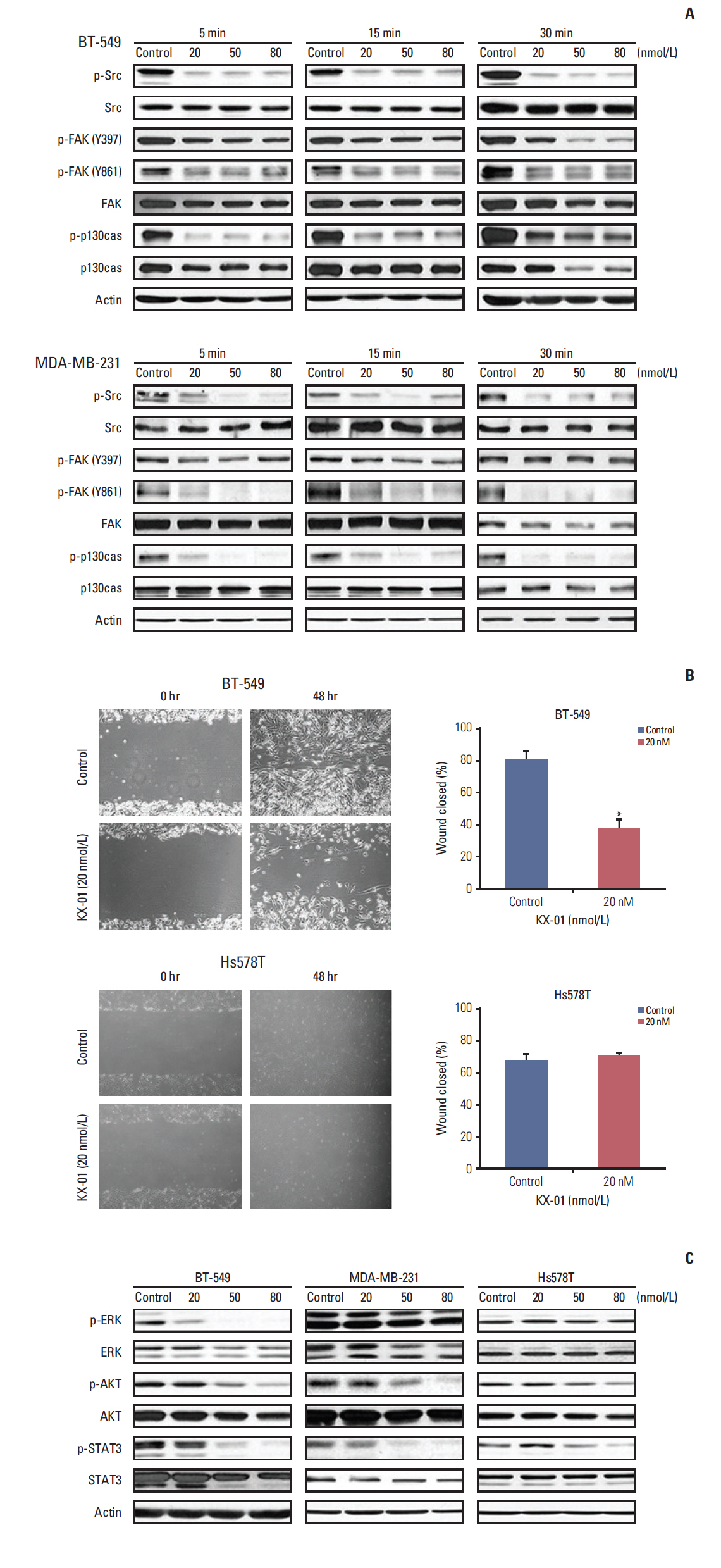
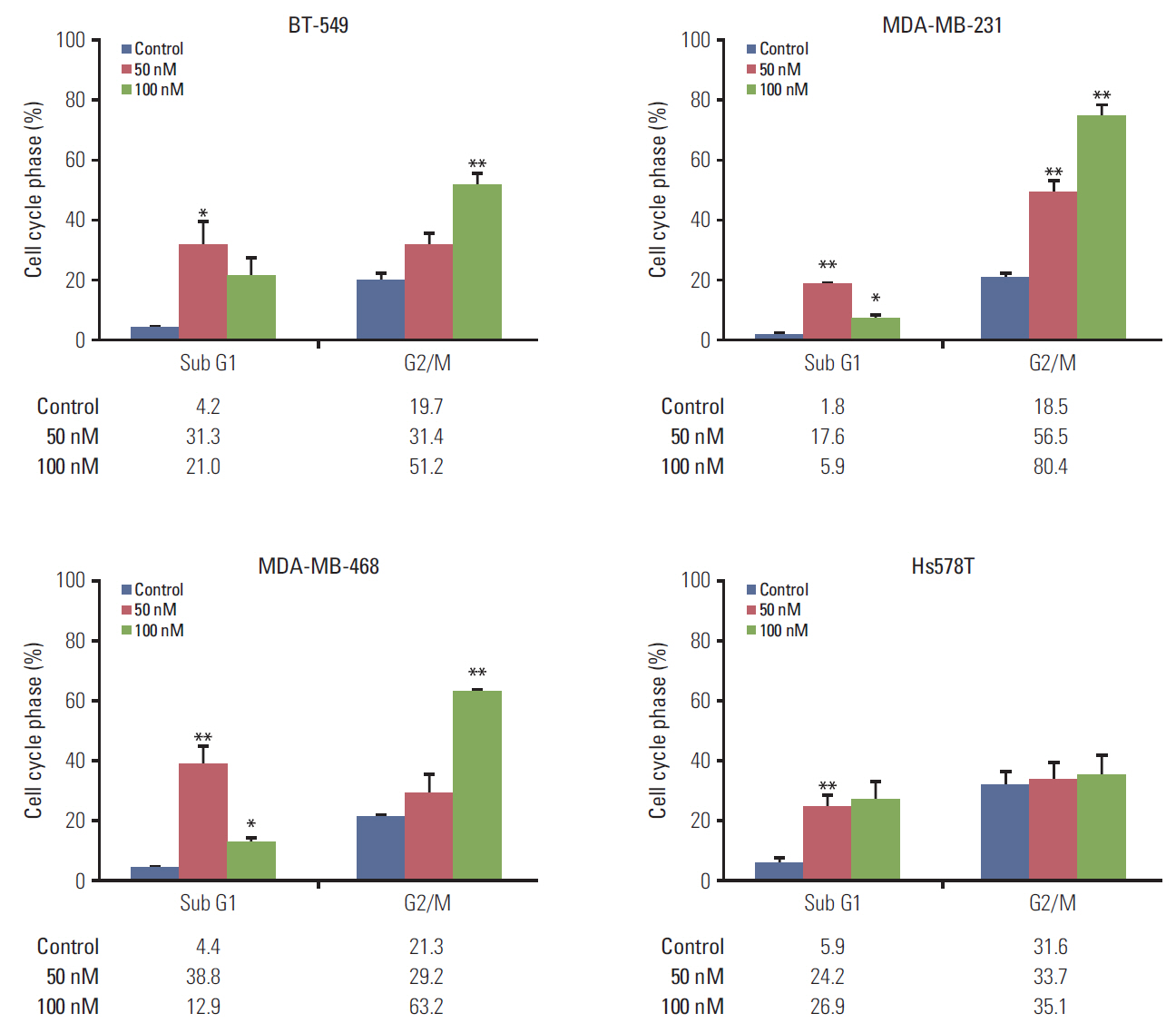
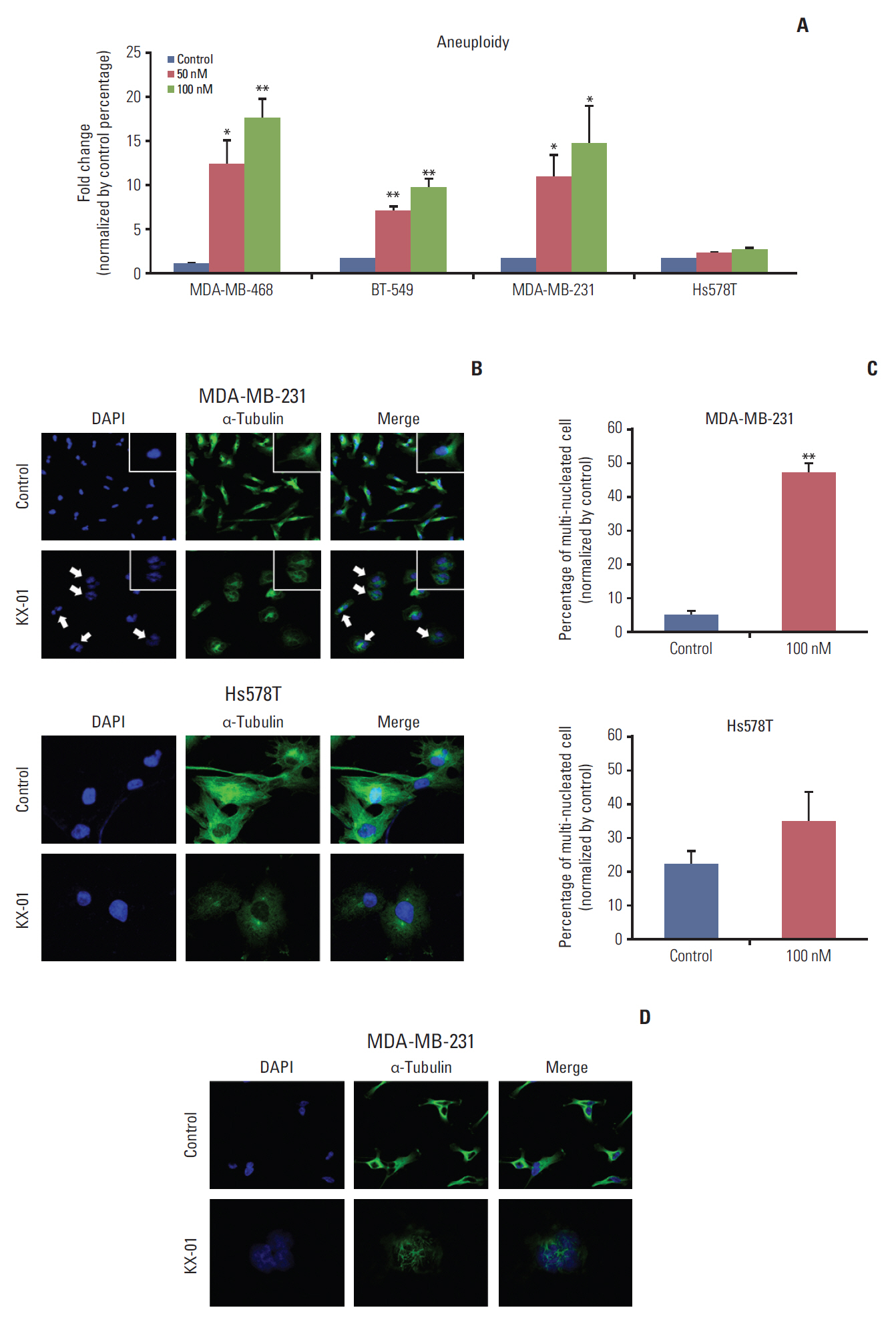
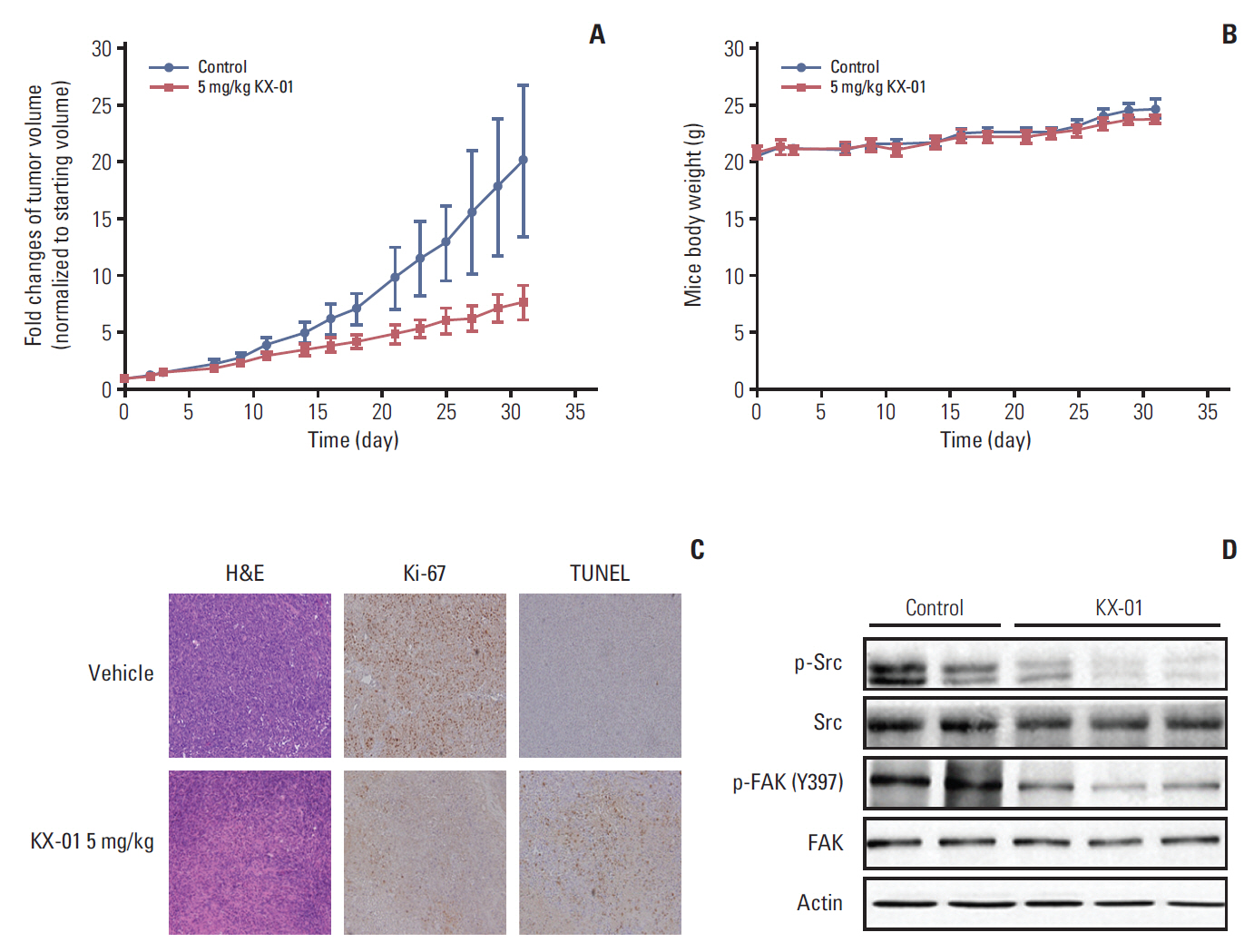
The antitumor effect of KX-01 in triple negative breast cancer (TNBC) cell lines was determined by MTT assay. Wound healing and immunofluorescence assays were performed to evaluate the action mechanisms of KX-01. Changes in the cell cycle and molecular changes induced by KX-01 were also evaluated. A MDA-MB-231 mouse xenograft model was used to demonstrate the in vivo effects.
RESULTS
KX-01 effectively inhibited the growth of breast cancer cell lines. The expression of phospho-Src and proliferative-signaling molecules were down-regulated in KX-01-sensitive TNBC cell lines. In addition, migration inhibition was observed by wound healing assay. KX-01-induced G2/M cell cycle arrest and increased the aneuploid cell population in KX-01-sensitive cell lines. Multi-nucleated cells were significantly increased after KX-01 treatment. Furthermore, KX-01 effectively delayed tumor growth in a MDA-MB-231 mouse xenograft model.
CONCLUSION
KX-01 effectively inhibited cell growth and migration of TNBC cells. Moreover, this study demonstrated that KX-01 showed antitumor effects through the inhibition of Src signaling and the induction of mitotic catastrophe. The antitumor effects of KX-01 were also demonstrated in vivo using a mouse xenograft model.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007; 8:235–44.
Article2. Tryfonopoulos D, Walsh S, Collins DM, Flanagan L, Quinn C, Corkery B, et al. Src: a potential target for the treatment of triple-negative breast cancer. Ann Oncol. 2011; 22:2234–40.
Article3. Hiscox S, Nicholson RI. Src inhibitors in breast cancer therapy. Expert Opin Ther Targets. 2008; 12:757–67.
Article4. Finn RS. Targeting Src in breast cancer. Ann Oncol. 2008; 19:1379–86.
Article5. Chen T, Pengetnze Y, Taylor CC. Src inhibition enhances paclitaxel cytotoxicity in ovarian cancer cells by caspase-9-independent activation of caspase-3. Mol Cancer Ther. 2005; 4:217–24.6. Finn RS, Bengala C, Ibrahim N, Roche H, Sparano J, Strauss LC, et al. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clin Cancer Res. 2011; 17:6905–13.
Article7. Gucalp A, Sparano JA, Caravelli J, Santamauro J, Patil S, Abbruzzi A, et al. Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin Breast Cancer. 2011; 11:306–11.
Article8. Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010; 7:683–92.
Article9. Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004; 23:2825–37.
Article10. Gardner MK, Zanic M, Howard J. Microtubule catastrophe and rescue. Curr Opin Cell Biol. 2013; 25:14–22.
Article11. Topham CH, Taylor SS. Mitosis and apoptosis: how is the balance set? Curr Opin Cell Biol. 2013; 25:780–5.
Article12. Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008; 15:1153–62.
Article13. Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011; 12:385–92.
Article14. Anbalagan M, Ali A, Jones RK, Marsden CG, Sheng M, Carrier L, et al. Peptidomimetic Src/pretubulin inhibitor KX-01 alone and in combination with paclitaxel suppresses growth, metastasis in human ER/PR/HER2-negative tumor xenografts. Mol Cancer Ther. 2012; 11:1936–47.
Article15. Anbalagan M, Carrier L, Glodowski S, Hangauer D, Shan B, Rowan BG. KX-01, a novel Src kinase inhibitor directed toward the peptide substrate site, synergizes with tamoxifen in estrogen receptor alpha positive breast cancer. Breast Cancer Res Treat. 2012; 132:391–409.16. Antonarakis ES, Heath EI, Posadas EM, Yu EY, Harrison MR, Bruce JY, et al. A phase 2 study of KX2-391, an oral inhibitor of Src kinase and tubulin polymerization, in men with bonemetastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2013; 71:883–92.
Article17. Naing A, Cohen R, Dy GK, Hong DS, Dyster L, Hangauer DG, et al. A phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket- directed SRC inhibitor, in patients with advanced malignancies. Invest New Drugs. 2013; 31:967–73.
Article18. Liu T, Hu W, Dalton HJ, Choi HJ, Huang J, Kang Y, et al. Targeting SRC and tubulin in mucinous ovarian carcinoma. Clin Cancer Res. 2013; 19:6532–43.
Article19. Kang S, Min A, Im SA, Song SH, Kim SG, Kim HA, et al. TGF-β suppresses COX-2 expression by tristetraprolin-mediated RNA destabilization in A549 human lung cancer cells. Cancer Res Treat. 2015; 47:101–9.
Article20. Min A, Im SA, Yoon YK, Song SH, Nam HJ, Hur HS, et al. RAD51C-deficient cancer cells are highly sensitive to the PARP inhibitor olaparib. Mol Cancer Ther. 2013; 12:865–77.
Article21. Nam HJ, Im SA, Oh DY, Elvin P, Kim HP, Yoon YK, et al. Antitumor activity of saracatinib (AZD0530), a c-Src/Abl kinase inhibitor, alone or in combination with chemotherapeutic agents in gastric cancer. Mol Cancer Ther. 2013; 12:16–26.
Article22. Macurek L, Draberova E, Richterova V, Sulimenko V, Sulimenko T, Draberova L, et al. Regulation of microtubule nucleation from membranes by complexes of membrane-bound gamma-tubulin with Fyn kinase and phosphoinositide 3-kinase. Biochem J. 2008; 416:421–30.23. Kasahara K, Nakayama Y, Nakazato Y, Ikeda K, Kuga T, Yamaguchi N. Src signaling regulates completion of abscission in cytokinesis through ERK/MAPK activation at the midbody. J Biol Chem. 2007; 282:5327–39.
Article24. Nakayama Y, Matsui Y, Takeda Y, Okamoto M, Abe K, Fukumoto Y, et al. c-Src but not Fyn promotes proper spindle orientation in early prometaphase. J Biol Chem. 2012; 287:24905–15.
Article25. Soeda S, Nakayama Y, Honda T, Aoki A, Tamura N, Abe K, et al. v-Src causes delocalization of Mklp1, Aurora B, and INCENP from the spindle midzone during cytokinesis failure. Exp Cell Res. 2013; 319:1382–97.
Article26. Park AY, Shen TL, Chien S, Guan JL. Role of focal adhesion kinase Ser-732 phosphorylation in centrosome function during mitosis. J Biol Chem. 2009; 284:9418–25.
Article27. Rea K, Sensi M, Anichini A, Canevari S, Tomassetti A. EGFR/MEK/ERK/CDK5-dependent integrin-independent FAK phosphorylated on serine 732 contributes to microtubule depolymerization and mitosis in tumor cells. Cell Death Dis. 2013; 4:e815.
Article28. Chee CE, Krishnamurthi S, Nock CJ, Meropol NJ, Gibbons J, Fu P, et al. Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. Oncologist. 2013; 18:1091–2.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Regulatory Mechanism of Src Familly Kinase in Lipopolysaccharide (LPS) induced HF-kappaB Activation Pathway
- Involvement of Src Family Tyrosine Kinase in Apoptosis of Human Neutrophils Induced by Protozoan Parasite Entamoeba histolytica
- Clathrin and Lipid Raft-dependent Internalization of Porphyromonas gingivalis in Endothelial Cells
- Metastasis-suppressor KAI1/CD82 induces homotypic aggregation of human prostate cancer cells through Src-dependent pathway
- Differentially Expressed Genes by Inhibition of C-terminal Src Kinase by siRNA in Human Vascular Smooth Muscle Cells and Their Association with Blood Pressure