Transl Clin Pharmacol.
2016 Mar;24(1):22-29. 10.12793/tcp.2016.24.1.22.
Development of a validated liquid chromatography-tandem mass spectrometry assay for the quantification of simvastatin acid, the active metabolite of simvastatin, in human plasma
- Affiliations
-
- 1Pharmacokinetic and Pharmacogenetic Laboratory, Clinical Research Center, Asan Medical Center, Pungnap-2-dong, Seoul, Republic of Korea.
- 2Department of Clinical Pharmacology, Inha University Hospital, Inha University School of Medicine, Incheon, Republic of Korea.
- 3Department of Pharmacology, Inje University College of Medicine, Busan, Republic of Korea.
- 4Division of Clinical Pharmacology, Clinical Trials Center, Pusan National University Hospital, Busan, Republic of Korea.
- 5Department of Anesthesiology, University of Ulsan College of Medicine, Asan Medical Center, Pungnap-2-dong, Seoul, Republic of Korea.
- 6Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan College of Medicine, Republic of Korea, Seoul, Republic of Korea. mdhslim@gmail.com
- KMID: 2383596
- DOI: http://doi.org/10.12793/tcp.2016.24.1.22
Abstract
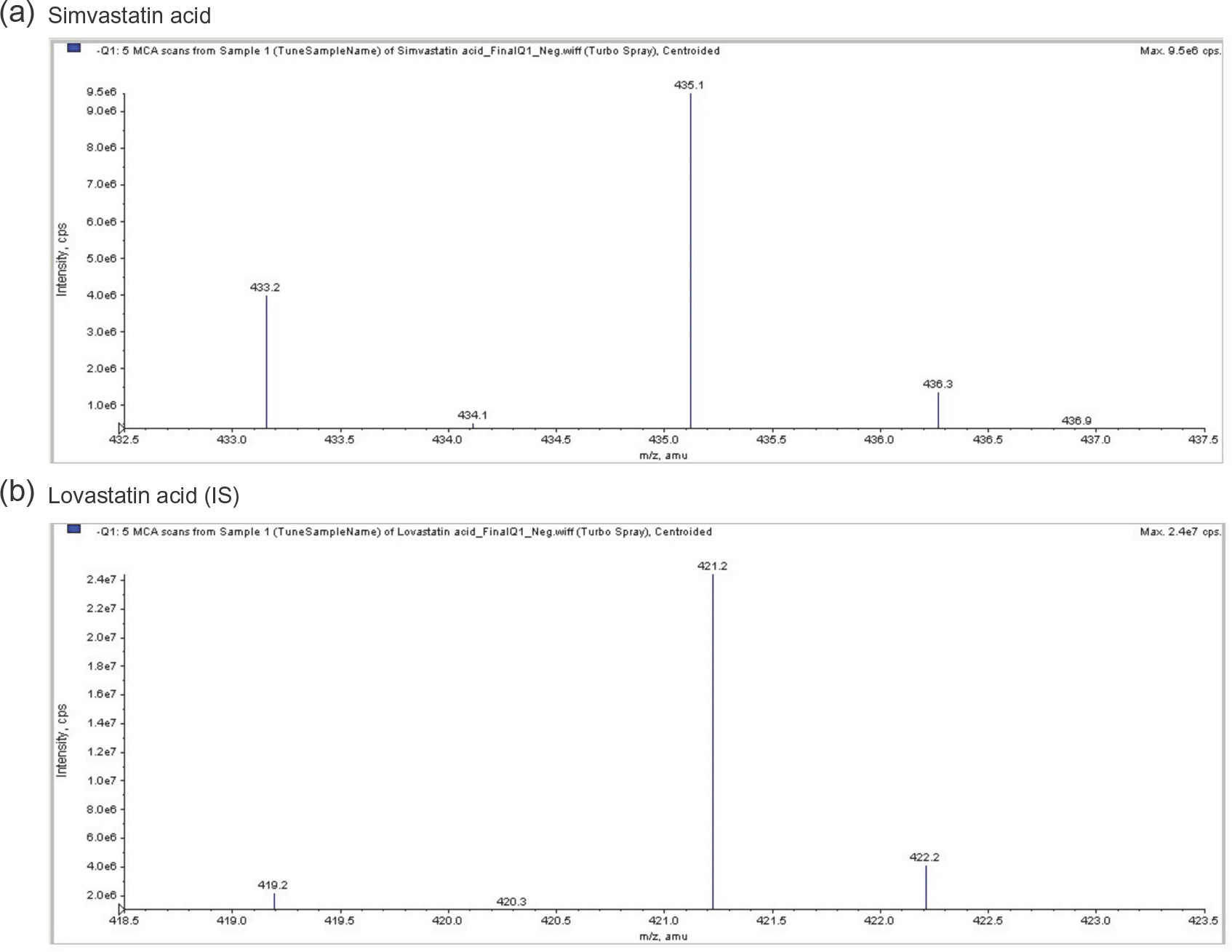
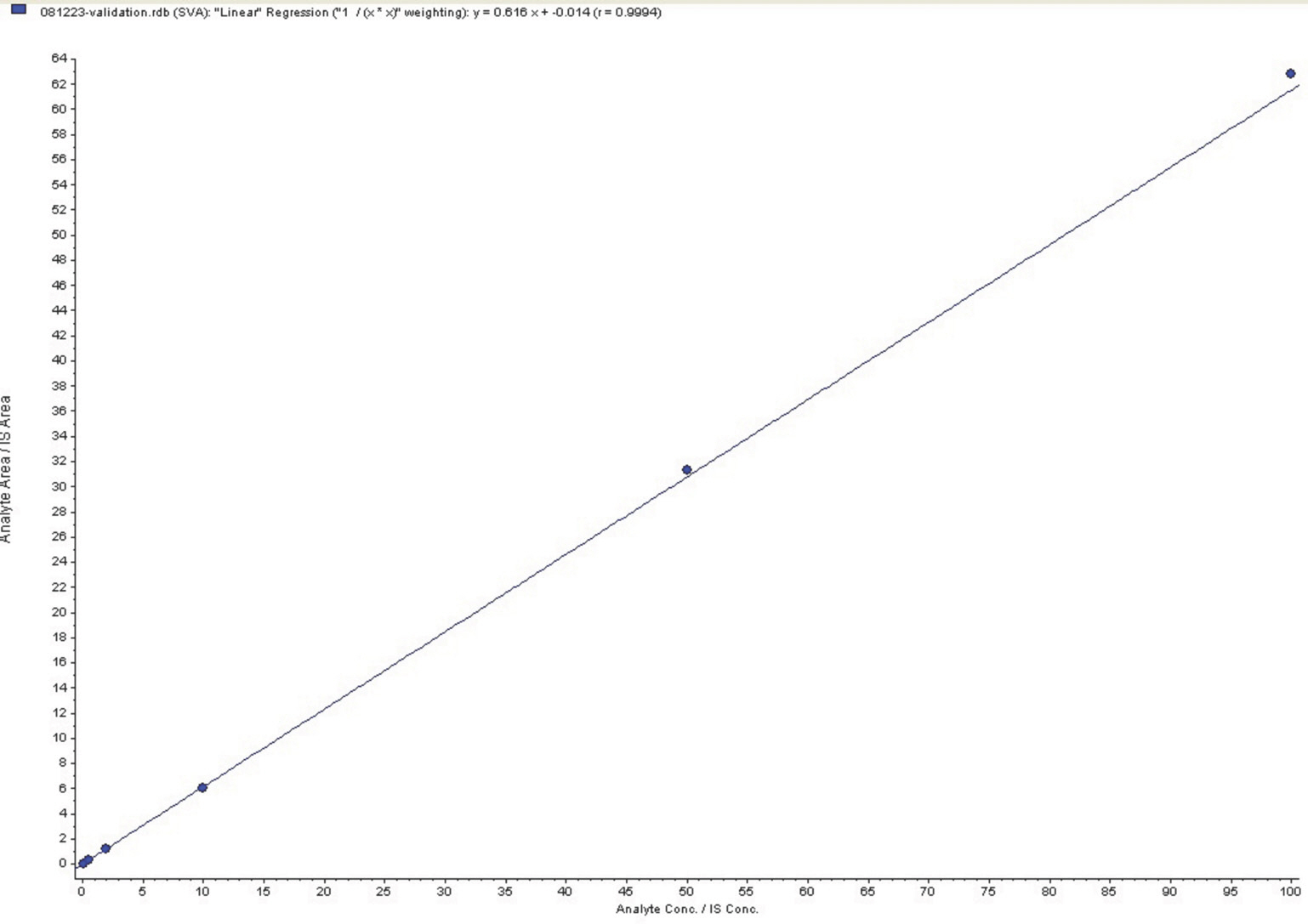
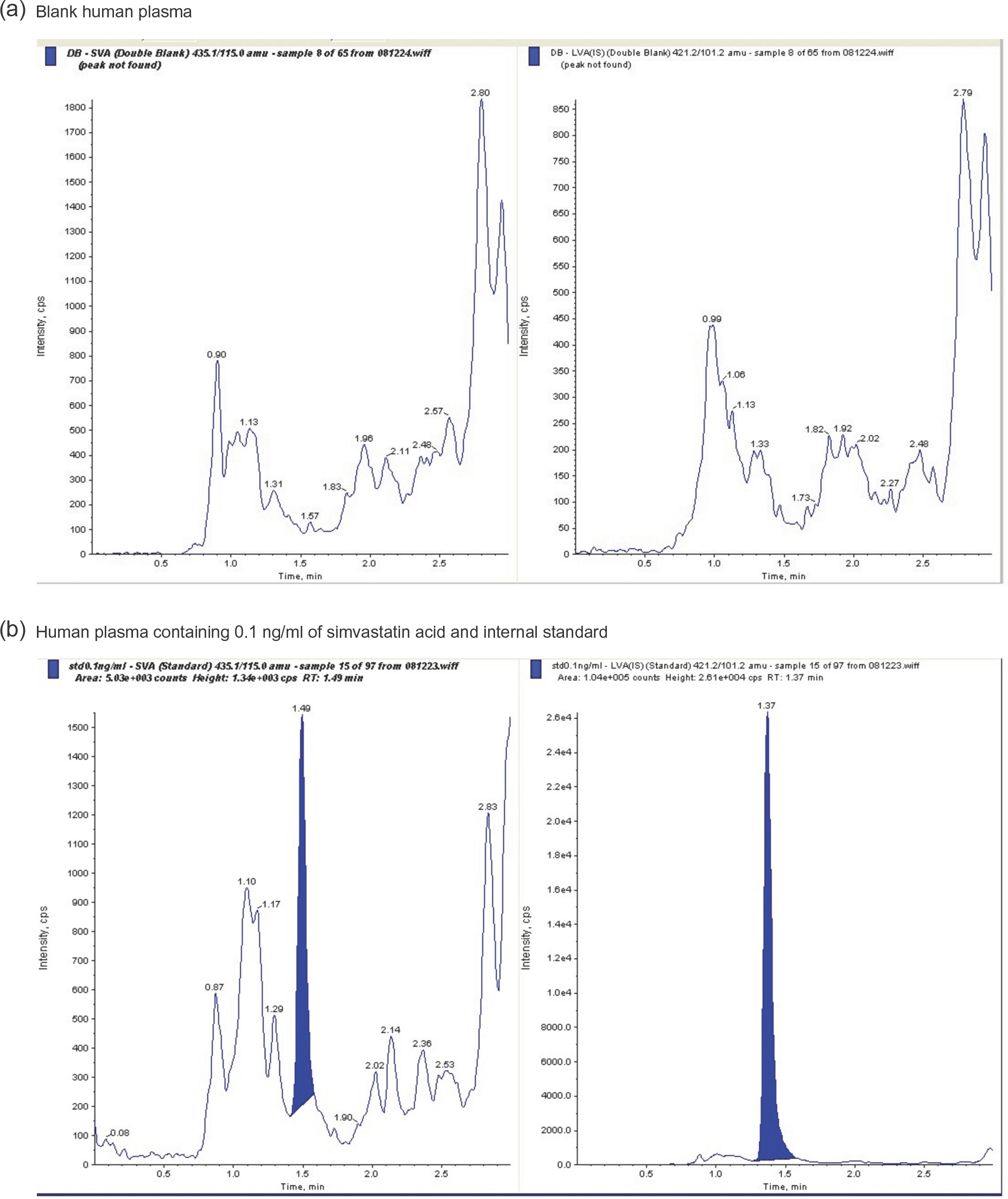
- Simvastatin is a lipid-lowering drug that is metabolized to its active metabolite simvastatin acid (SA). We developed and validated a sensitive liquid chromatography-tandem mass spectrometry (LC/MS/MS) method to quantitate SA in human plasma using a liquid-liquid extraction method with methanol. The protonated analytes generated in negative ion mode were monitored by multiple reaction monitoring. Using 500-mL plasma aliquots, SA was quantified in the range of 0.1-100 ng/mL. Calibration was performed by internal standardization with lovastatin acid, and regression curves were generated using a weighting factor of 1/χ2. The linearity, precision, and accuracy of this assay for each compound were validated using quality control samples consisting of mixtures of SA (0.1, 0.5, 5, and 50 ng/mL) and plasma. The intra-batch accuracy was 95.3-107.8%, precision was -2.2% to -3.7%, and linearity (r2) was over 0.998 in the standard calibration range. The chromatographic running time was 3.0 min. This method sensitively and reliably measured SA concentrations in human plasma and was successfully used in clinical pharmacokinetic studies of simvastatin in healthy Korean adult male volunteers.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994; 344:1383–1389.2. HMG-CoA reductase inhibitors for hypercholesterolemia. N Engl J Med. 1988; 319:1222–1223.3. Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013; 1:CD004816.
Article4. Minder CM, Blaha MJ, Horne A, Michos ED, Kaul S, Blumenthal RS. Evidence-based use of statins for primary prevention of cardiovascular disease. Am J Med. 2012; 125:440–446. doi: 10.1016/j.amjmed.2011.11.013.
Article5. Prueksaritanont T, Gorham LM, Ma B, Liu L, Yu X, Zhao JJ, et al. In vitro metabolism of simvastatin in humans [SBT] identification of metabolizing enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos. 1997; 25:1191–1199.6. Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol. 2003; 56:120–124.
Article7. Matusewicz L, Meissner J, Toporkiewicz M, Sikorski AF. The effect of statins on cancer cells–review. Tumour Biol. 2015 Jul; 36:4889–4904. doi: 10. 1007/s13277-015-3551-7.
Article8. Merck Sharpe & Dohme. Manufacturer Information, Zocor: simvastatin, West Point, PA. 1991.9. Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH, et al. Glucuronidation of statins in animals and humans: a novel mechanism of statin lactonization. Drug Metab Dispos. 2002; 30:505–512.
Article10. Prueksaritanont T, Qiu Y, Mu L, Michel K, Brunner J, Richards KM, et al. Interconversion pharmacokinetics of simvastatin and its hydroxy acid in dogs: effects of gemfibrozil. Pharm Res. 2005; 22:1101–1109.
Article11. Jemal M, Ouyang Z, Powell ML. Direct-injection LC-MS-MS method for high-throughput simultaneous quantitation of simvastatin and simvastatin acid in human plasma. J Pharm Biomed Anal. 2000; 23:323–340.
Article12. Jin SJ, Bae KS, Cho SH, Jung JA, Kim U, Choe S, et al. Population pharmacokinetic analysis of simvastatin and its active metabolite with the characterization of atypical complex absorption kinetics. Pharm Res. 2004; 31:1801–1812. doi: 10.1007/s11095-013-1284-0.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Simultaneous quantification of ticagrelor and its active metabolite, AR-C124910XX, in human plasma by liquid chromatography-tandem mass spectrometry: Applications in steady-state pharmacokinetics in patients
- A Novel Simultaneous Determination of Sarpogrelate and its Active Metabolite (M-1) in Human Plasma, Using Liquid Chromatography-Tandem Mass Spectrometry: Clinical Application
- Development and Validation of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of epsilon-Acetamidocaproic Acid in Rat Plasma
- Determination of donepezil in human plasma using ultra performance liquid chromatography-tandem mass spectrometry
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry