Transl Clin Pharmacol.
2019 Sep;27(3):98-106. 10.12793/tcp.2019.27.3.98.
Simultaneous quantification of ticagrelor and its active metabolite, AR-C124910XX, in human plasma by liquid chromatography-tandem mass spectrometry: Applications in steady-state pharmacokinetics in patients
- Affiliations
-
- 1College of Pharmacy and Integrated Research Institute of Pharmaceutical Sciences, The Catholic University of Korea, Bucheon 14662, Republic of Korea. baesk@catholic.ac.kr
- 2Department of Pharmacy and Yonsei Institute of Pharmaceutical Sciences, College of Pharmacy, Yonsei University, Incheon 21983, Republic of Korea.
- KMID: 2458949
- DOI: http://doi.org/10.12793/tcp.2019.27.3.98
Abstract
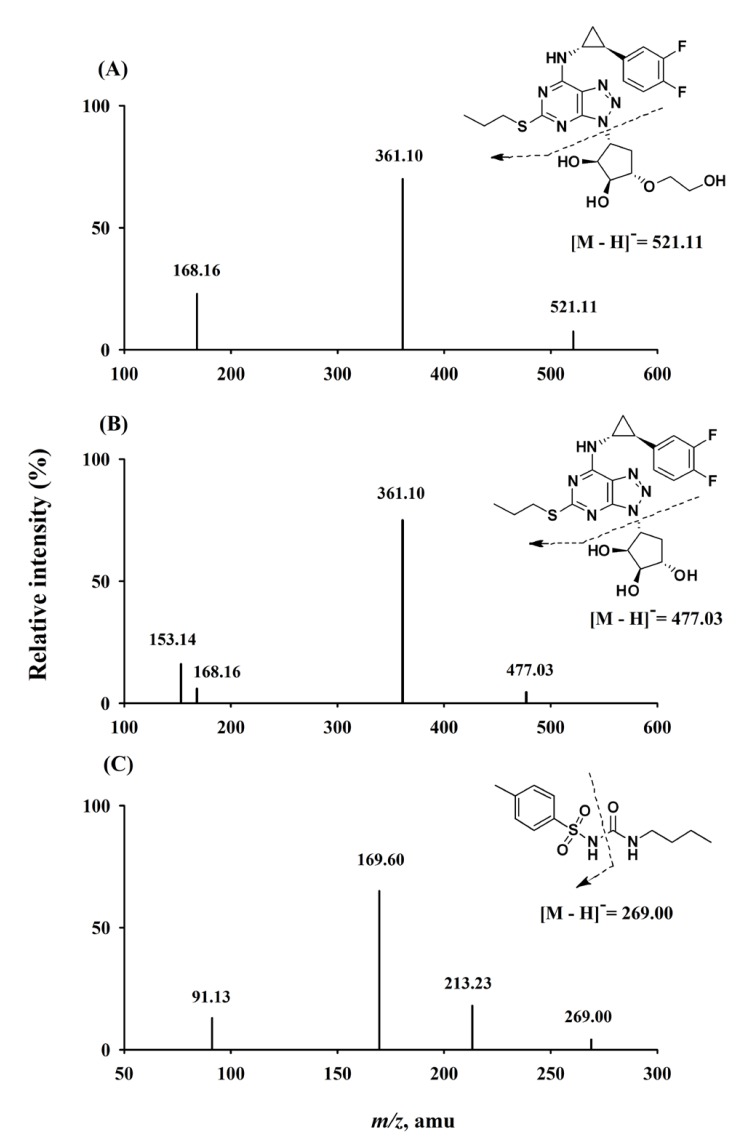
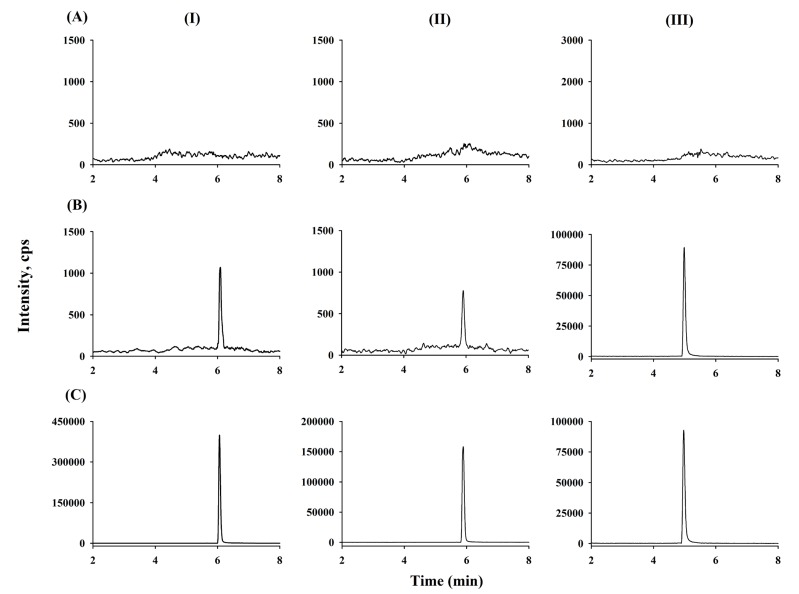
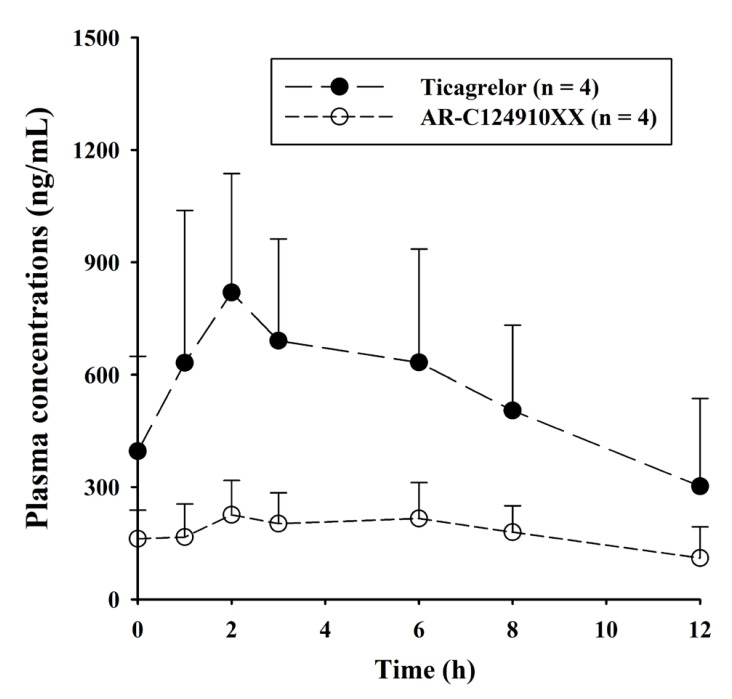
- A sensitive and simple liquid chromatography-tandem mass spectrometry method was developed and validated for the simultaneous quantification of ticagrelor and its active metabolite, AR-C124910XX from 50 µL human plasma using tolbutamide as an internal standard as per regulatory guidelines. Analytes in plasma were extracted by simple protein precipitation using acetonitrile, followed by chromatographic separation with an Acclaimâ„¢ RSLC 120 Câ‚₈ column (2.2 µm, 2.1 × 100 mm) and a gradient acetonitrile-water mobile phase containing 0.1% formic acid within 8 min. Mass spectrometric detection and quantitation were conducted by selected reaction-monitoring on a negative electrospray ionization mode with the following transitions: m/z 521.11 → 361.10, 477.03 → 361.10, and 269.00 → 169.60 for ticagrelor, AR-C124910XX, and tolbutamide, respectively. The lower limit of quantifications was 0.2 ng/mL with linear ranges of 0.2-2,500 ng/mL (r²â‰¥ 0.9949) for both analytes. All validation data, including selectivity, cross-talk, precision, accuracy, matrix effect, recovery, dilution integrity, stability, and incurred sample reanalysis, were well within acceptable limits. This assay method was validated using Kâ‚‚-EDTA as the specific anticoagulant. Also, the anticoagulant effect was tested by lithium heparin, sodium heparin, and K₃-EDTA. No relevant anticoagulant effect was observed. This validated method was effectively used in the determination of ticagrelor and its active metabolite, AR-C124910XX, in plasma samples from patients with myocardial infarction.
Keyword
MeSH Terms
Figure
Reference
-
1. Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009; 27:259–274. DOI: 10.1111/j.1755-5922.2009.00096.x. PMID: 19604248.2. Springthorpe B, Bailey A, Barton P, Birkinshaw TN, Bonnert RV, Brown RC, et al. From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett. 2007; 17:6013–6018. DOI: 10.1016/j.bmcl.2007.07.057. PMID: 17827008.
Article3. Li P, Gu Y, Yang Y, Chen L, Liu J, Gao L, et al. Low-dose ticagrelor yields an antiplatelet efficacy similar to that of standard-dose ticagrelor in healthy subjects: an open-label randomized controlled trial. Sci Rep. 2016; 6:31838. DOI: 10.1038/srep31838. PMID: 27554803.
Article4. Orme RC, Parker WAE, Thomas MR, Judge HM, Baster K, Sumaya W, et al. Study of two dose regimens of ticagrelor compared with clopidogrel in patients undergoing percutaneous coronary intervention for stable coronary artery disease (STEEL-PCI). Circulation. 2018; [In Press]. DOI: 10.1161/CIRCULATIONAHA.118.034790.5. Xu Q, Sun Y, Zhang Y, Liu B, Fang L, Shen C, et al. Effect of a 180 mg ticagrelor loading dose on myocardial necrosis in patients undergoing elective percutaneous coronary intervention: A preliminary study. Cardiol J. 2017; 24:15–24. DOI: 10.5603/CJ.a2017.0002. PMID: 28070882.
Article6. Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010; 38:1514–1521. DOI: 10.1124/dmd.110.032250. PMID: 20551239.
Article7. Zhou D, Andersson TB, Grimm SW. In vitro evaluation of potential drug-drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction, and differential kinetics. Drug Metab Dispos. 2011; 39:703–710. DOI: 10.1124/dmd.110.037143. PMID: 21177984.
Article8. Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: A double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006; 27:1038–1047. DOI: 10.1093/eurheartj/ehi754. PMID: 16476694.
Article9. Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y(12) receptor antagonist, in healthy subjects. Eur J Clin Pharmacol. 2010; 66:487–496. DOI: 10.1007/s00228-009-0778-5. PMID: 20091161.
Article10. Sillén H, Cook M, Davis P. Determination of unbound ticagrelor and its active metabolite (AR-C124910XX) in human plasma by equilibrium dialysis and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011; 879:2315–2322. DOI: 10.1016/j.jchromb.2011.06.023.
Article11. Danielak D, Gorzycka P, Kruszyna Ł, Karaźniewicz-Łada M, Główka F. Development of an LC-MS/MS method for simultaneous determination of ticagrelor and its active metabolite during concomitant treatment with atorvastatin. J Chromatogr B Analyt Technol Biomed Life Sci. 2019; 1105:113–119. DOI: 10.1016/j.jchromb.2018.12.018.
Article12. Peng J, Wang Y, Li M, He C, Chen Y, Chen X, et al. An HPLC-MS/MS method for the quantitative determination of ticagrelor and its active metabolite AR-C124910XX in human plasma and its application to a clinical study. Curr Pharma Anal. 2017; 13:296–303. DOI: 10.2174/1573412912666160518151458.
Article13. Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:2299–2306. DOI: 10.1016/j.jchromb.2010.06.018.
Article14. Xu X, Ding X, Yuan B, Li W, Wang Y, Jin Y, et al. Validated liquid chromatography-tandem mass spectrometry method for quantification of ticagrelor and its active metabolite in human plasma. Biomed Chromatogr. 2019; 33:e4498. DOI: 10.1002/bmc.4498. PMID: 30675914.
Article15. Zhong W, Wang X, Tang L, Mai L, Chen XP, He G, et al. Simultaneous determination of ticagrelor and its metabolites in human plasma and urine using liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 2016; 40:445–453. DOI: 10.1093/jat/bkw039. PMID: 27165805.
Article16. Lee YJ, Kim H, Choi J, Lee BH, Lee SY. Evaluation of pharmacokinetic, pharmacodynamic, efficacy, and safety data of low-dose ticagrelor versus standard dose in East Asians: A systematic review. Ther Clin Risk Manag. 2018; 14:83–93. DOI: 10.2147/TCRM.S152276. PMID: 29386900.
Article17. Li H, Guo J, Carlson GF, Teng R. Pharmacodynamics, pharmacokinetics, and safety of ticagrelor in Chinese patients with stable coronary artery disease. Br J Clin Pharmacol. 2016; 82:352–361. DOI: 10.1111/bcp.12950. PMID: 27038001.
Article18. Park DW, Lee PH, Jang S, Lim HS, Kang DY, Lee CH, et al. Effect of low-dose versus standard-dose ticagrelor and clopidogrel on platelet Inhibition in acute coronary syndromes. J Am Coll Cardiol. 2018; 71:1594–1595. DOI: 10.1016/j.jacc. PMID: 29622168.
Article19. Guidance for Industry-Bioanalytical method validation (2013) US Food and Drug Administration. Rockville, MD, USA: Accessed 8 May 2019. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.20. Liang HR, Foltz RL, Meng M, Bennett P. Ionization enhancement in atmospheric pressure chemical ionization and suppression in electrospray ionization between target drugs and stable-isotope-labeled internal standards in quantitative liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003; 17:2815–2821. DOI: 10.1002/rcm.1268. PMID: 14673832.
Article21. Remane D, Wissenbach DK, Meyer MR, Maurer HH. Systematic investigation of ion suppression and enhancement effects of fourteen stable-isotope-labeled internal standards by their native analogues using atmospheric-pressure chemical ionization and electrospray ionization and the relevance for multi-analyte liquid chromatographic/mass spectrometric procedures. Rapid Commun Mass Spectrom. 2010; 24:859–867. DOI: 10.1002/rcm.4459. PMID: 20196193.22. Wang S, Cyronak M, Yang E. Does a stable isotopically labeled internal standard always correct analyte response? A matrix effect study on a LC/MS/MS method for the determination of carvedilol enantiomers in human plasma. J Pharm Biomed Anal. 2007; 43:701–707. DOI: 10.1016/j.jpba.2006.08.010. PMID: 16959461.23. Srinivas NR. Should commonly prescribed drugs be avoided as internal standard choices in new assays for clinical samples? Bioanalysis. 2016; 8:607–610. DOI: 10.4155/bio.16.21. PMID: 26964873.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Novel Simultaneous Determination of Sarpogrelate and its Active Metabolite (M-1) in Human Plasma, Using Liquid Chromatography-Tandem Mass Spectrometry: Clinical Application
- Development and Validation of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of epsilon-Acetamidocaproic Acid in Rat Plasma
- Validation of LC-MS/MS Method for Determination of Bivalirudin in Human Plasma: Application to a Pharmacokinetic Study
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry
- Determination of donepezil in human plasma using ultra performance liquid chromatography-tandem mass spectrometry