Ann Lab Med.
2015 Jul;35(4):391-398. 10.3343/alm.2015.35.4.391.
A Novel Simultaneous Determination of Sarpogrelate and its Active Metabolite (M-1) in Human Plasma, Using Liquid Chromatography-Tandem Mass Spectrometry: Clinical Application
- Affiliations
-
- 1Clinical Trial Center, Clinical Research Institute, Samsung Medical Center, Korea.
- 2Department of Clinical Pharmacology and Therapeutics, Samsung Medical Center, Korea. suddenbz@skku.edu
- 3Department of Clinical Research Design and Evaluation, SAIHST, Sungkyunkwan University, Korea.
- 4Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2045860
- DOI: http://doi.org/10.3343/alm.2015.35.4.391
Abstract
- BACKGROUND
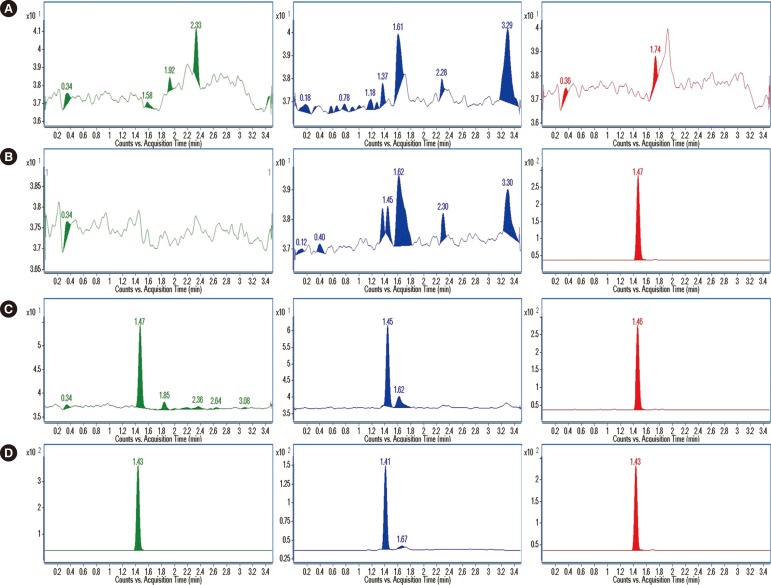
This study describes a novel analytical method for simultaneously determining sarpogrelate and its metabolite (M-1) in human plasma, using liquid chromatography coupled with tandem mass spectrometry, with electrospray ionization in the positive ion mode.
METHODS
Sarpogrelate, M-1, and labeled internal standard (d3-sarpogrelate) were extracted from 50 microL of human plasma by simple protein precipitation. Chromatographic separation was performed by using a linear gradient elution of a mobile phase involving water-formic acid (99.9:0.1, v/v) and acetonitrile-formic acid (99.9:0.1, v/v) over 4 min of run time on a column, with a core-shell-type stationary phase (Kinetex C18, 50 mm x 2.1 mm i.d., 2.6-microm particle size, Phenomenex, USA). Detection of the column effluent was performed by using a triple-quadruple mass spectrometer in the multiple-reaction monitoring mode.
RESULTS
The developed method was validated in human plasma, with lower limits of quantification of 10 ng/mL for sarpogrelate and 2 ng/mL for M-1. The calibration curves of sarpogrelate and M-1 were linear over the concentration ranges of 10-2,000 and 2-400 ng/mL, respectively (R2>0.99). The carry-over effect, precision, accuracy, and stability of the method met the criteria for acceptance.
CONCLUSIONS
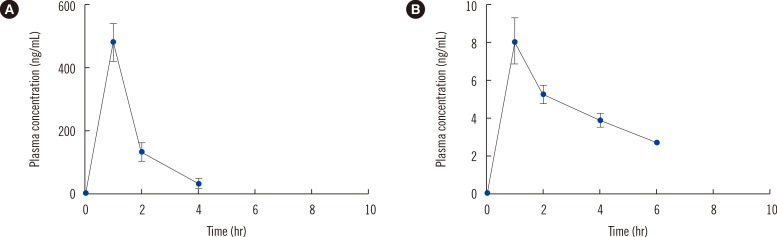
A simple, fast, robust, and reliable analytical method was successfully developed and applied to the high-throughput determination of sarpogrelate and its metabolite in real plasma samples in a pharmacokinetic study of healthy subjects.
Keyword
MeSH Terms
Figure
Reference
-
1. Muntasir HA, Bhuiyan MA, Ishiguro M, Ozaki M, Nagatomo T. Inverse agonist activity of sarpogrelate, a selective 5-HT2A-receptor antagonist, at the constitutively active human 5-HT2A receptor. J Pharmacol Sci. 2006; 102:189–195. PMID: 17031071.
Article2. Ogawa T, Sugidachi A, Tanaka N, Fujimoto K, Asai F. Pharmacological profiles of R-96544, the active form of a novel 5-HT2A receptor antagonist R-102444. Eur J Pharmacol. 2002; 457:107–114. PMID: 12464356.
Article3. Israilova M, Suzuki F, Tanaka T, Nagatomo T, Taniguchi T, Muramatsu I. Binding and functional affinity of sarpogrelate, its metabolite m-1 and ketanserin for human recombinant alpha-1-adrenoceptor subtypes. Pharmacology. 2002; 65:69–73. PMID: 11937776.
Article4. Orlandini E, Rapposelli S, Nencetti S, Giannaccini G, Betti L, Balsamo A. Synthesis and 5-HT2A, 5-HT1A and alpha1-binding affinities of 2-[2-Hydroxy-3-(pyridin-3-yl-methyl)amino]-, 2-[2-hydroxy-3-(2-pyridin-2-yl-ethyl)amino]- and 2-[2-hydroxy-3-(4-N-methyl-piperazin-1-yl)-amino]propoxybenzaldehyde-O-(substituted) benzyl oximes. Arch Pharm (Weinheim). 2007; 340:135–139. PMID: 17335104.5. Saini HK, Takeda N, Goyal RK, Kumamoto H, Arneja AS, Dhalla NS. Therapeutic potentials of sarpogrelate in cardiovascular disease. Cardiovasc Drug Rev. 2004; 22:27–54. PMID: 14978517.
Article6. Uchiyama S, Ozaki Y, Satoh K, Kondo K, Nishimaru K. Effect of sarpogrelate, a 5-HT(2A) antagonist, on platelet aggregation in patients with ischemic stroke: clinical-pharmacological dose-response study. Cerebrovasc Dis. 2007; 24:264–270. PMID: 17622759.7. Patankar S, Pudage A, Joshi SS, Vaidya VV, Gomes NA. Rapid and sensitive LC-MS-MS method for the determination of sarpogrelate in human plasma. Chromatographia. 2009; 69:671–676.
Article8. Nirogi R, Kandikere V, Mudigonda K, Ajjala D, Suraneni R, Thoddi P. Liquid chromatography tandem mass spectrometry method for the quantification of sarpogrelate, a selective 5-HT2A receptor antagonist, in plasma: application to a pre-clinical pharmacokinetic study. Biomed Chromatogr. 2010; 24:1159–1167. PMID: 20954206.9. Zhang C, Wang L, Yang Y, Sun Y, Zhang J, Li G, et al. Validated LC-MS/MS method for the determination of sarpogrelate in human plasma: application to a pharmacokinetic and bioequivalence study in Chinese volunteers. J Pharm Biomed Anal. 2010; 53:546–551. PMID: 20399588.
Article10. Kim TE, Kim JR, Jung JA, Kim SR, Lee JW, Jun H, et al. Comparison of pharmacokinetics between sarpogrelate hydrochloride immediate-release formulation and controlled-release formulation. Int J Clin Pharmacol Ther. 2013; 51:114–119. PMID: 23073143.
Article11. US Food and Drug Administration. Guidance for industry: Bioanalytical method validation. Updated on Sep 2013. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM368107.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Simultaneous quantification of ticagrelor and its active metabolite, AR-C124910XX, in human plasma by liquid chromatography-tandem mass spectrometry: Applications in steady-state pharmacokinetics in patients
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry
- Determination of donepezil in human plasma using ultra performance liquid chromatography-tandem mass spectrometry
- Development and Validation of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of epsilon-Acetamidocaproic Acid in Rat Plasma
- Development of a validated liquid chromatography-tandem mass spectrometry assay for the quantification of simvastatin acid, the active metabolite of simvastatin, in human plasma