Int J Stem Cells.
2015 Nov;8(2):155-169. 10.15283/ijsc.2015.8.2.155.
Cryopreservation of Human Wharton's Jelly-derived Mesenchymal Stem Cells Following Controlled Rate Freezing Protocol Using Different Cryoprotectants; A Comparative Study
- Affiliations
-
- 1Department of Vet OBS/Theriogenology and Biotechnology, Gyeongsang National University, Jinju, Korea. jinrho@gnu.ac.kr
- 2Department of Orthopaedic Surgery, School of Medicine, Gyeongsang National University, Jinju, Korea.
- 3Department of Obstetrics and Gynaecology, School of Medicine, Gyeongsang National University, Jinju, Korea.
- 4Department of Oral and Maxillofacial Surgery, School of Medicine, Gyeongsang National University, Jinju, Korea. parkbw@gnu.ac.kr
- 5Research Institute of Life Sciences, Gyeongsang National University, Jinju, Korea.
- KMID: 2380810
- DOI: http://doi.org/10.15283/ijsc.2015.8.2.155
Abstract
OBJECTIVES
To compare the effect of three different cryoprotectants on basic stem cell characteristics for the possibility of using well defined, dimethyl sulfoxide (DMSO) and serum free freezing solutions to cryopreserve human Wharton's jelly-derived mesenchymal stem cells (WJMSCs) following controlled rate freezing protocol.
METHODS
The mesenchymal stem cells isolated from human Wharton's jelly were cryopreserved using 10% DMSO, 10% polyvinylpyrrolidone (PVP) and a cocktail solution comprising of 0.05 M glucose, 0.05 M sucrose and 1.5 M ethylene glycol following controlled rate freezing protocol. We investigated the post-thaw cell viability, morphology, proliferation capacity, basic stem cell characteristics, in vitro differentiation potential and apoptosis-related gene expression profile before and after cryopreservation.
RESULTS
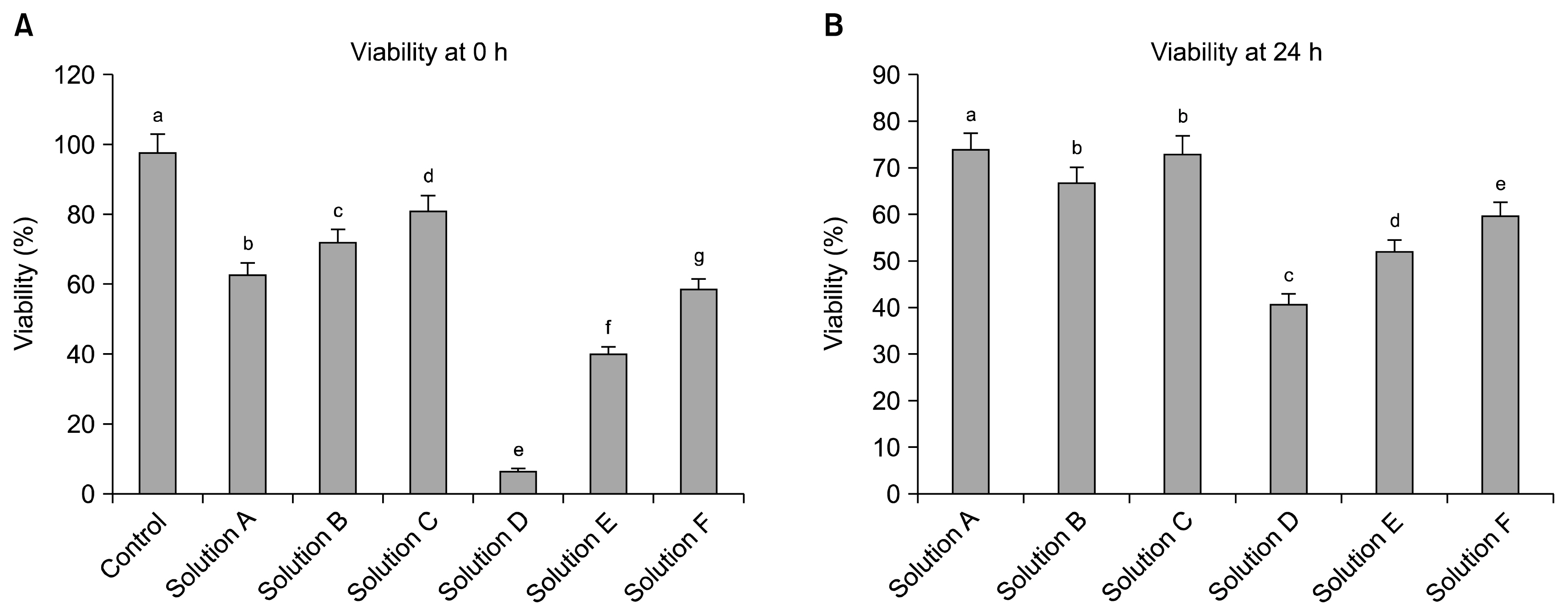
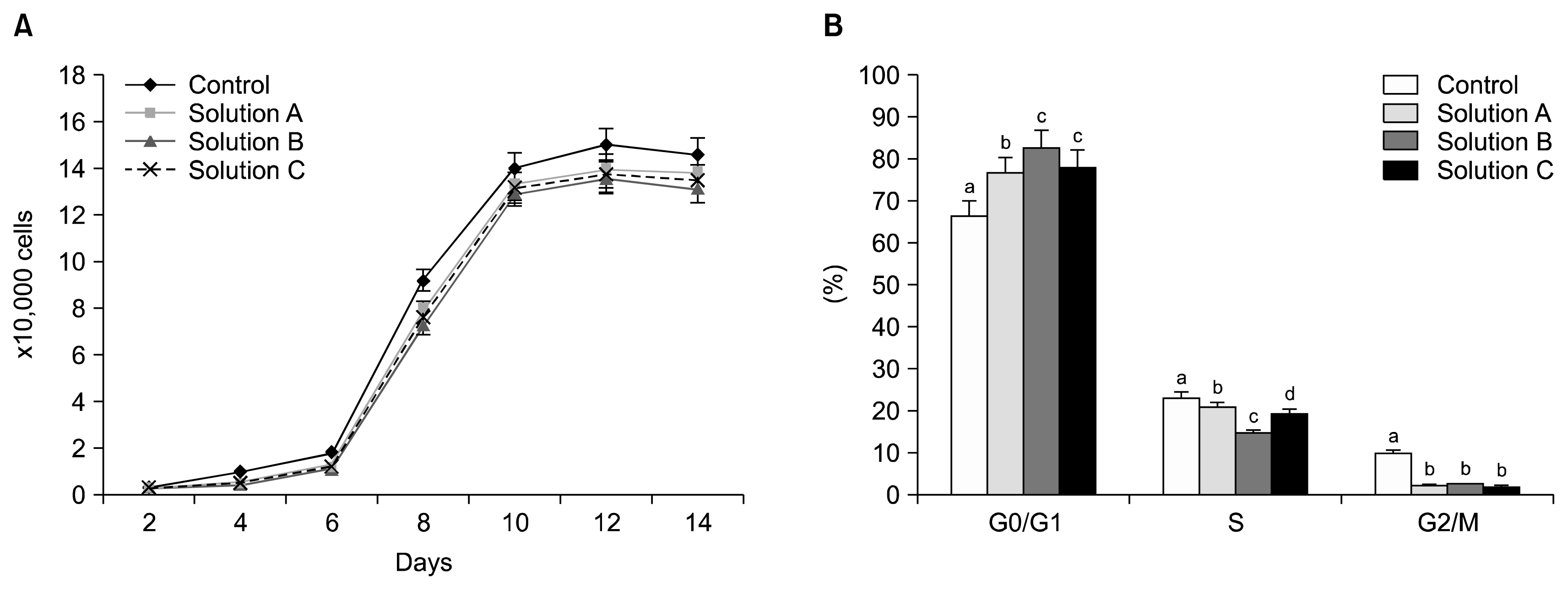
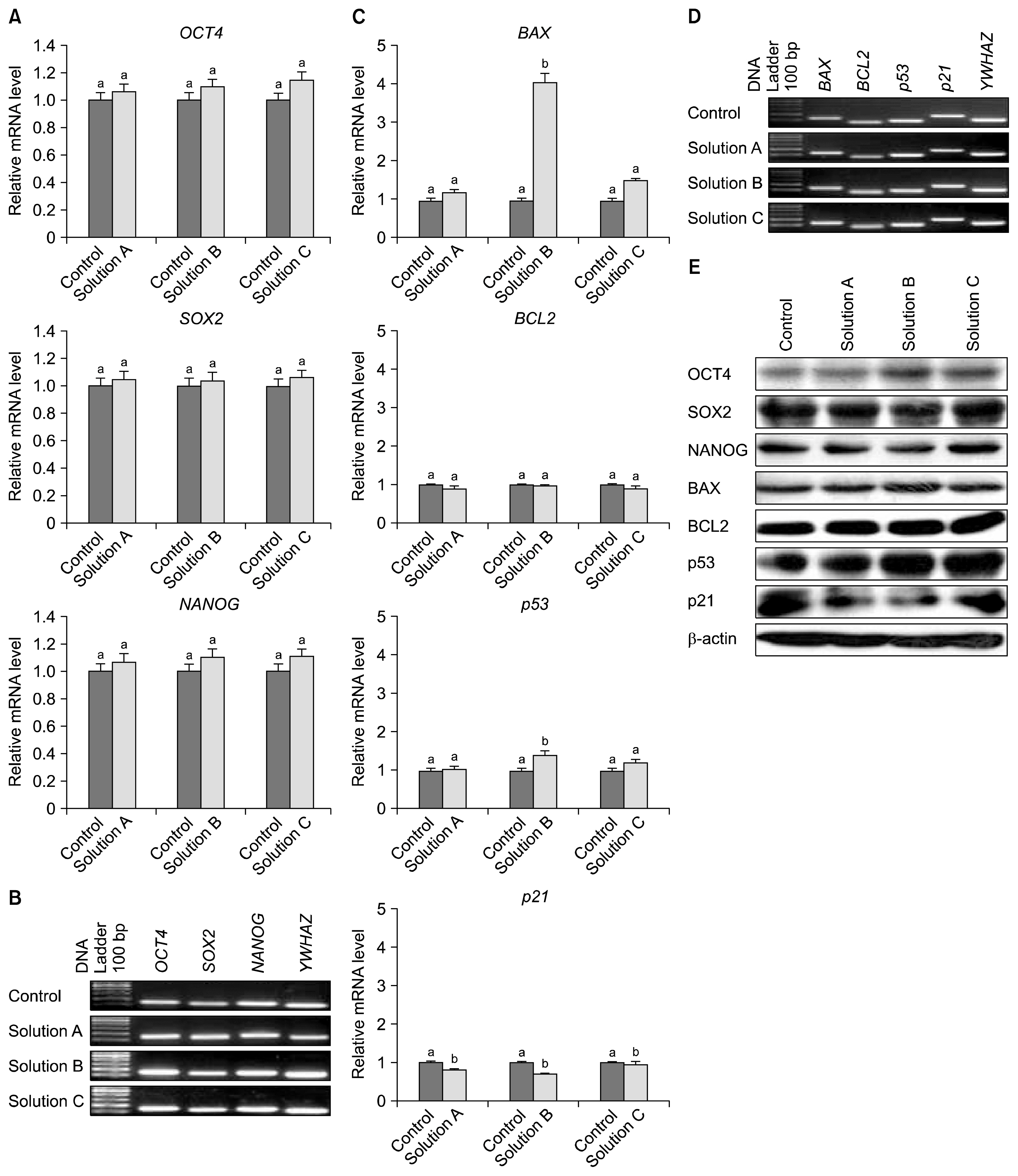
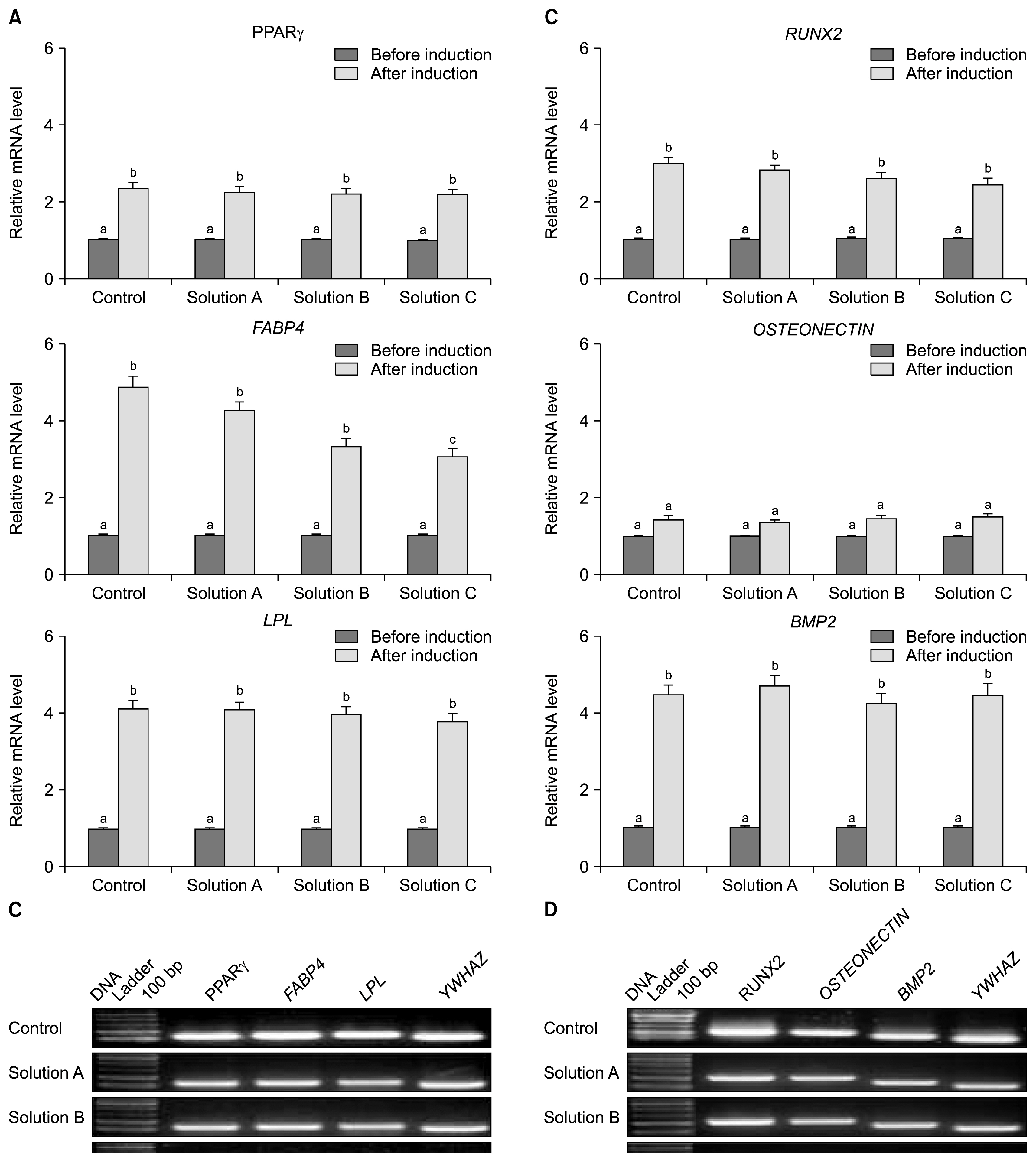
The cryoprotectant 10% DMSO has shown higher post-thaw cell viability of 81.2+/-0.58% whereas 10% PVP and cocktail solution have shown 62.87+/-0.35% and 72.2+/-0.23%, respectively at 0 h immediately thawing. The cell viability was further reduced in all the cryopreserved groups at 24 h later post-thaw culture. Further, the complete elimination of FBS in cryoprotectants has resulted in drastic reduction in cell viability. Cryopreservation did not alter the basic stem cell characteristics, plasticity and multipotency except proliferation rate. The expression of pro-apoptotic BAX and p53 genes were higher whilst p21 was lower in all the cryopreserved groups when compare to the control group of WJMSCs.
CONCLUSION
Although 10% DMSO has shown higher post-thaw cell viability compare to 10% PVP and cocktail solution, the present study indicates the feasibility of developing a well-defined DMSO free cryosolution which can improve storage and future broad range applications of WJMSCs in regenerative medicine without losing their basic stem cell characteristics.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976; 4:267–274. PMID: 976387.2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.
Article3. Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004; 40:1275–1284. DOI: 10.1002/hep.20469. PMID: 15562440.
Article4. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61:364–370. DOI: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. PMID: 10931522.
Article5. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119:2204–2213. DOI: 10.1242/jcs.02932. PMID: 16684817.
Article6. Abdulrazzak H, Moschidou D, Jones G, Guillot PV. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface. 2010; 7(Suppl 6):S689–706. DOI: 10.1098/rsif.2010.0347.focus. PMID: 20739312. PMCID: 2988276.
Article7. Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells. 2010; 2:81–92. DOI: 10.4252/wjsc.v2.i4.81.
Article8. Sabapathy V, Sundaram B, VMS , Mankuzhy P, Kumar S. Human Wharton’s Jelly Mesenchymal Stem Cells plasticity augments scar-free skin wound healing with hair growth. PLoS One. 2014; 9:e93726. DOI: 10.1371/journal.pone.0093726.
Article9. Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011; 9:12. DOI: 10.1186/1478-811X-9-12. PMID: 21569606. PMCID: 3117820.
Article10. Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005; 23:220–229. DOI: 10.1634/stemcells.2004-0166. PMID: 15671145.
Article11. McElreavey KD, Irvine AI, Ennis KT, McLean WH. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem Soc Trans. 1991; 19:29S. DOI: 10.1042/bst019029s.
Article12. Taghizadeh RR, Cetrulo KJ, Cetrulo CL. Wharton’s Jelly stem cells: future clinical applications. Placenta. 2011; 32(Suppl 4):S311–S315. DOI: 10.1016/j.placenta.2011.06.010.
Article13. Wang XY, Lan Y, He WY, Zhang L, Yao HY, Hou CM, Tong Y, Liu YL, Yang G, Liu XD, Yang X, Liu B, Mao N. Identification of mesenchymal stem cells in aorta-gonad-mesonephros and yolk sac of human embryos. Blood. 2008; 111:2436–2443. DOI: 10.1182/blood-2007-07-099333.
Article14. Brockbank KGM, Covault JC, Taylor MJ. Cryopreservation manual: a guide to cryopreservation techniques. Mariette, USA: Thermo Farma Sci. Publ. Gr;2001.15. Pegg DE. Principles of cryopreservation. Day JG, Stacey G, editors. Cryopreservation and freeze-drying protocols. 2nd ed. New Jersey: Humana Press Inc;2007. p. 39–74. DOI: 10.1007/978-1-59745-362-2_3.
Article16. Massood E, Maryam K, Parvin S, Mojgan M, Noureddin NM. Vitrification of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells. Cryo Letters. 2013; 34:471–480.17. Luciano AM, Chigioni S, Lodde V, Franciosi F, Luvoni GC, Modina SC. Effect of different cryopreservation protocols on cytoskeleton and gap junction mediated communication integrity in feline germinal vesicle stage oocytes. Cryobiology. 2009; 59:90–95. DOI: 10.1016/j.cryobiol.2009.05.002. PMID: 19460364.
Article18. Balci D, Can A. The assessment of cryopreservation conditions for human umbilical cord stroma-derived mesenchymal stem cells towards a potential use for stem cell banking. Curr Stem Cell Res Ther. 2013; 8:60–72. DOI: 10.2174/1574888X11308010008.
Article19. Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Letters. 2004; 25:375–388.20. Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008; 3:e1451. DOI: 10.1371/journal.pone.0001451.
Article21. Thirumala S, Wu X, Gimble JM, Devireddy RV. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng Part C Methods. 2010; 16:783–792. DOI: 10.1089/ten.tec.2009.0552.
Article22. Park BW, Jang SJ, Byun JH, Kang YH, Choi MJ, Park WU, Lee WJ, Rho GJ. Cryopreservation of human dental follicle tissue for use as a resource of autologous mesenchymal stem cells. J Tissue Eng Regen Med. 2014; DOI: 10.1002/term.1945. [Epub ahead of print]. PMID: 25052907.
Article23. Subbarao RB, Ullah I, Kim EJ, Jang SJ, Lee WJ, Jeon RH, Kang D, Lee SL, Park BW, Rho GJ. Characterization and evaluation of neuronal trans-differentiation with electrophysiological properties of mesenchymal stem cells isolated from porcine endometrium. Int J Mol Sci. 2015; 16:10934–10951. DOI: 10.3390/ijms160510934. PMID: 26006231. PMCID: 4463684.
Article24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001; 25:402–408. DOI: 10.1006/meth.2001.1262.
Article25. Ruiz-Delgado GJ, Mancías-Guerra C, Tamez-Gómez EL, Rodríguez-Romo LN, López-Otero A, Hernández-Arizpe A, Gómez-Almaguer D, Ruiz-Argüelles GJ. Dimethyl sulf-oxide-induced toxicity in cord blood stem cell transplantation: report of three cases and review of the literature. Acta Haematol. 2009; 122:1–5. DOI: 10.1159/000227267.
Article26. Ock SA, Rho GJ. Effect of dimethyl sulfoxide (DMSO) on cryopreservation of porcine mesenchymal stem cells (pMSCs). Cell Transplant. 2011; 20:1231–1239. DOI: 10.3727/096368910X552835. PMID: 21294964.
Article27. Liu Y, Xu X, Ma X, Martin-Rendon E, Watt S, Cui Z. Cryopreservation of human bone marrow-derived mesenchymal stem cells with reduced dimethylsulfoxide and well-defined freezing solutions. Biotechnol Prog. 2010; 26:1635–1643. DOI: 10.1002/btpr.464. PMID: 20572296.
Article28. Heng BC. Effect of Rho-associated kinase (ROCK) inhibitor Y-27632 on the post-thaw viability of cryopreserved human bone marrow-derived mesenchymal stem cells. Tissue Cell. 2009; 41:376–380. DOI: 10.1016/j.tice.2009.01.004. PMID: 19261317.
Article29. Sundin M, Ringdén O, Sundberg B, Nava S, Götherström C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007; 92:1208–1215. DOI: 10.3324/haematol.11446. PMID: 17666368.
Article30. Ha SY, Jee BC, Suh CS, Kim HS, Oh SK, Kim SH, Moon SY. Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Hum Reprod. 2005; 20:1779–1785. DOI: 10.1093/humrep/deh854. PMID: 15760949.
Article31. Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014; 34:695–704. PMID: 24970492. PMCID: 4121354.
Article32. Kuleshova LL, Tan FC, Magalhães R, Gouk SS, Lee KH, Dawe GS. Effective cryopreservation of neural stem or progenitor cells without serum or proteins by vitrification. Cell Transplant. 2009; 18:135–144. DOI: 10.3727/096368909788341298. PMID: 19499702.
Article33. Naaldijk Y, Fedorova V, Stolzing A. Cryopreservation of human umbilical cord-derived mesenchymal stem cells in complex sugar based cryoprotective solutions. J Biotechnol Lett. 2013; 4:95–99.34. Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M. Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010; 19:117–130. DOI: 10.1089/scd.2009.0177.
Article35. Roy S, Arora S, Kumari P, Ta M. A simple and serum-free protocol for cryopreservation of human umbilical cord as source of Wharton’s jelly mesenchymal stem cells. Cryobiolog. 2014; 68:467–472. DOI: 10.1016/j.cryobiol.2014.03.010.
Article36. Heng BC, Ye CP, Liu H, Toh WS, Rufaihah AJ, Yang Z, Bay BH, Ge Z, Ouyang HW, Lee EH, Cao T. Loss of viability during freeze-thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. J Biomed Sci. 2006; 13:433–445. DOI: 10.1007/s11373-005-9051-9.
Article37. Pucci B, Kasten M, Giordano A. Cell cycle and apoptosis. Neoplasia. 2000; 2:291–299. DOI: 10.1038/sj.neo.7900101. PMID: 11005563. PMCID: 1550296.
Article38. Schneider E, Montenarh M, Wagner P. Regulation of CAK kinase activity by p53. Oncogene. 1998; 17:2733–2741. DOI: 10.1038/sj.onc.1202504. PMID: 9840937.
Article39. Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990; 348:334–336. DOI: 10.1038/348334a0. PMID: 2250705.
Article40. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–619. DOI: 10.1016/0092-8674(93)90509-O. PMID: 8358790.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- Cardioprotective Effects of Wharton Jelly Derived Mesenchymal Stem Cell Transplantation in a Rodent Model of Myocardial Injury
- Viability and Colony Forming Capacity of Hematopoietic Stem Cells after Cryopreservation
- Differentiation of human male germ cells from Wharton's jelly-derived mesenchymal stem cells
- Mesenchymal Stem Cells from the Wharton's Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential