Int J Stem Cells.
2019 Jul;12(2):218-226. 10.15283/ijsc18034.
Mesenchymal Stem Cells from the Wharton's Jelly of the Human Umbilical Cord: Biological Properties and Therapeutic Potential
- Affiliations
-
- 1Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy. mantocastaldi@msn.com
- 2Department of Obstetrics and Gynecology, Azienda Ospedaliera di Rilievo Nazionale e di Alta Specialità “San Giuseppe Moscatiâ€, Avellino, Italy.
- 3Orthopedic Biotechnology Laboratory, Galeazzi Orthopedic Institute, Milan, Italy.
- KMID: 2465893
- DOI: http://doi.org/10.15283/ijsc18034
Abstract
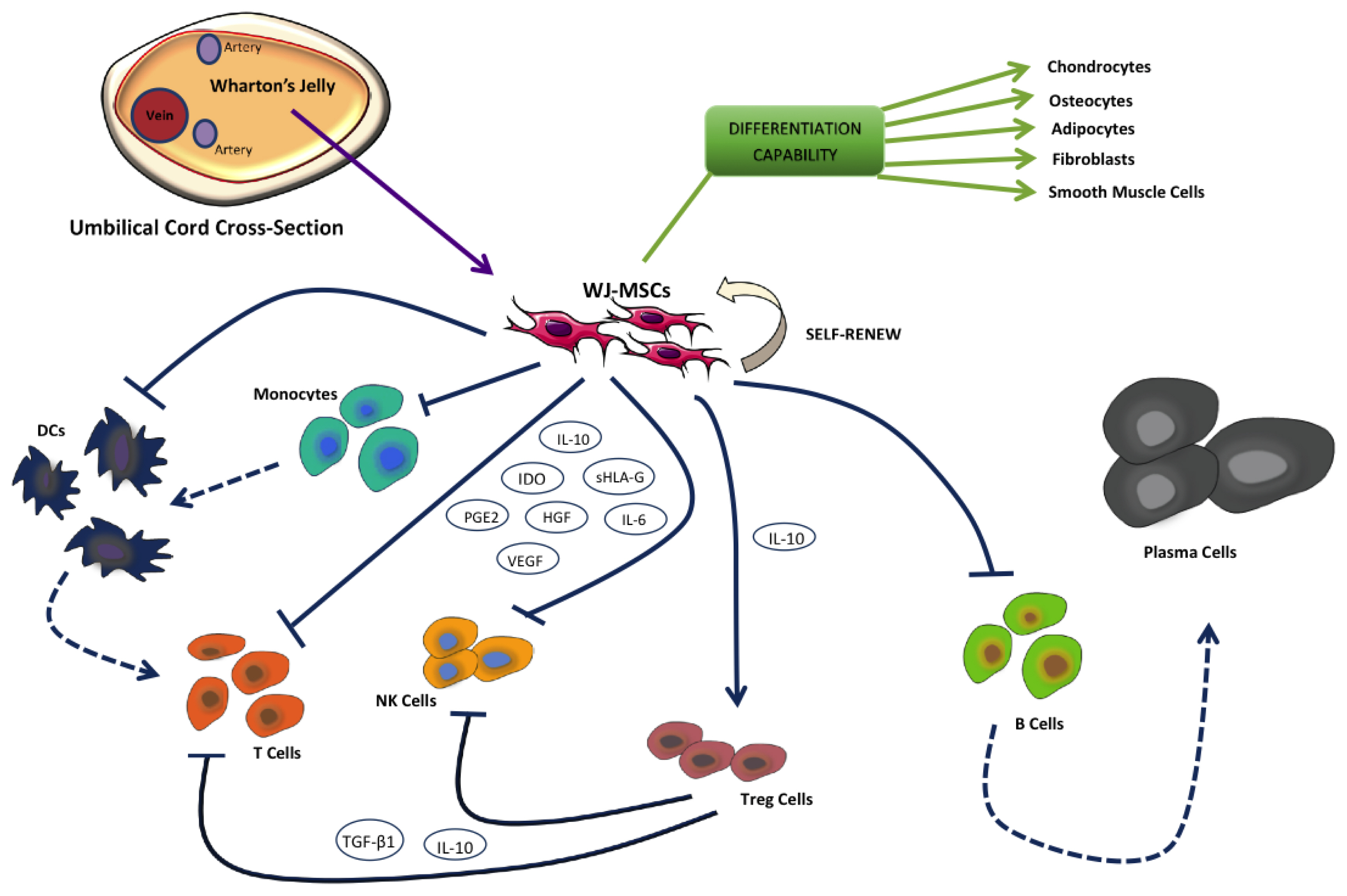
- Wharton's jelly mesenchymal stem cells (WJ-MSCs) are a class of stem cells with high differentiative potential, an immuno-privileged status and easy access for collection, which raise no legal or ethical issues. WJ-MSCs exhibit several features of embryonic stem cells, both in the phenotypic and genetic aspects, with only a few differences, such as a shorter doubling time and a more extensive ex vivo expansion capacity. WJ-MSCs have immunomodulatory properties, involving both innate and adaptive immune responses. This review focuses on the role of WJ-MSCs in the management of graft-versus-host disease (GvHD), a life-threatening complication of the allogenic transplantation of hematopoietic stem cells. Different studies documented the beneficial effect of the infusion of WJ-MSCs, even when not fully HLA identical, in patients with severe GvHD, refractory to standard treatment. Finally, we summarized current ongoing clinical trials with WJ-MSCs and their potential in regenerative medicine.
MeSH Terms
Figure
Reference
-
References
1. Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974; 17:331–340. DOI: 10.1097/00007890-197404000-00001. PMID: 4150881.
Article2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.
Article3. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119:2204–2213. DOI: 10.1242/jcs.02932. PMID: 16684817.
Article4. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. DOI: 10.1080/14653240600855905. PMID: 16923606.
Article5. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13:4279–4295. DOI: 10.1091/mbc.e02-02-0105. PMID: 12475952. PMCID: PMC138633.
Article6. Abdulrazzak H, Moschidou D, Jones G, Guillot PV. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface. 2010; 7(Suppl 6):S689–706. DOI: 10.1098/rsif.2010.0347.focus. PMID: 20739312. PMCID: PMC2988276.
Article7. Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008; 26:591–599. DOI: 10.1634/stemcells.2007-0439. PMID: 18065397. PMCID: PMC3311226.8. Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003; 33:919–926. DOI: 10.1016/j.bone.2003.07.005. PMID: 14678851.
Article9. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O. Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008; 371:1579–1586. DOI: 10.1016/S0140-6736(08)60690-X. PMID: 18468541.
Article10. McElreavey KD, Irvine AI, Ennis KT, McLean WH. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem Soc Trans. 1991; 19:29S. DOI: 10.1042/bst019029s. PMID: 1709890.
Article11. Kuroda Y, Kitada M, Wakao S, Dezawa M. Mesenchymal stem cells and umbilical cord as sources for schwann cell differentiation: their potential in peripheral nerve repair. Open Tissue Eng Regen Med J. 2011; 4:54–63. DOI: 10.2174/1875043501104010054.
Article12. Du T, Zou X, Cheng J, Wu S, Zhong L, Ju G, Zhu J, Liu G, Zhu Y, Xia S. Human Wharton’s jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem Cell Res Ther. 2013; 4:59. DOI: 10.1186/scrt215. PMID: 23734757. PMCID: PMC3706832.
Article13. Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R, Trounson A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009; 175:303–313. DOI: 10.2353/ajpath.2009.080629. PMID: 19497992. PMCID: PMC2708816.
Article14. Lo Iacono M, Anzalone R, Corrao S, Giuffrè M, Di Stefano A, Giannuzzi P, Cappello F, Farina F, La Rocca G. Perinatal and Wharton’s jelly-derived mesenchymal stem cells in cartilage regenerative medicine and tissue engineering strategies. Open Tissue Eng Regen Med J. 2011; 4:72–81. DOI: 10.2174/1875043501104010072.
Article15. Scheers I, Lombard C, Najimi M, Sokal EM. Cell therapy for the treatment of metabolic liver disease: an update on the umbilical cord derived stem cells candidates. Open Tissue Eng Regen Med J. 2011; 4:48–53. DOI: 10.2174/1875043501104010048.
Article16. Tamura M, Kawabata A, Ohta N, Uppalapati L, Becker KG, Troyer D. Wharton’s jelly stem cells as agents for cancer therapy. Open Tissue Eng Regen Med J. 2011; 4:39–47. DOI: 10.2174/1875043501104010039.
Article17. Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T, Phan TT, Volk HD, Reichenspurner H, Robbins RC, Schrepfer S. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant. 2011; 20:655–667. DOI: 10.3727/096368910X536473. PMID: 21054940.
Article18. Zhou C, Yang B, Tian Y, Jiao H, Zheng W, Wang J, Guan F. Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol. 2011; 272:33–38. DOI: 10.1016/j.cellimm.2011.09.010. PMID: 22004796. PMCID: PMC3235326.
Article19. Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+ CD25highFOXP3+ regulatory T cells. Stem Cells. 2008; 26:212–222. DOI: 10.1634/stemcells.2007-0554. PMID: 17932417.
Article20. Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008; 26:2865–2874. DOI: 10.1634/stemcells.2007-1028. PMID: 18703664.
Article21. Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 2010; 5:e9016. DOI: 10.1371/journal.pone.0009016. PMID: 20126406. PMCID: PMC2814860.22. La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasà L, Cappello F, Zummo G, Farina F. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009; 131:267–282. DOI: 10.1007/s00418-008-0519-3. PMID: 18836737.
Article23. Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noël D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007; 25:2025–2032. DOI: 10.1634/stemcells.2006-0548. PMID: 17510220.
Article24. Pontikoglou C, Deschaseaux F, Sensebé L, Papadaki HA. Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. 2011; 7:569–589. DOI: 10.1007/s12015-011-9228-8. PMID: 21249477.
Article25. Tipnis S, Viswanathan C, Majumdar AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol. 2010; 88:795–806. DOI: 10.1038/icb.2010.47. PMID: 20386557.
Article26. Che N, Li X, Zhou S, Liu R, Shi D, Lu L, Sun L. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol. 2012; 274:46–53. DOI: 10.1016/j.cellimm.2012.02.004. PMID: 22414555.
Article27. Ribeiro A, Laranjeira P, Mendes S, Velada I, Leite C, Andrade P, Santos F, Henriques A, Grãos M, Cardoso CM, Martinho A, Pais M, da Silva CL, Cabral J, Trindade H, Paiva A. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013; 4:125. DOI: 10.1186/scrt336. PMID: 24406104. PMCID: PMC3854702.
Article28. Nekanti U, Mohanty L, Venugopal P, Balasubramanian S, Totey S, Ta M. Optimization and scale-up of Wharton’s jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010; 5:244–254. DOI: 10.1016/j.scr.2010.08.005. PMID: 20880767.
Article29. Fong CY, Richards M, Manasi N, Biswas A, Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod Biomed Online. 2007; 15:708–718. DOI: 10.1016/S1472-6483(10)60539-1. PMID: 18062871.
Article30. Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M. Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010; 19:117–130. DOI: 10.1089/scd.2009.0177. PMID: 19619003.
Article31. Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006; 24:781–792. DOI: 10.1634/stemcells.2005-0330. PMID: 16223852.
Article32. Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007; 25:319–331. DOI: 10.1634/stemcells.2006-0286. PMID: 17053211.
Article33. Pavletic SZ, Fowler DH. Are we making progress in GVHD prophylaxis and treatment? Hematology Am Soc Hematol Educ Program. 2012; 2012:251–264. PMID: 23233589.
Article34. Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012; 12:443–458. DOI: 10.1038/nri3212. PMID: 22576252. PMCID: PMC3552454.
Article35. Deeg HJ. How I treat refractory acute GVHD. Blood. 2007; 109:4119–4126. DOI: 10.1182/blood-2006-12-041889. PMID: 17234737. PMCID: PMC1885485.
Article36. Wu KH, Chan CK, Tsai C, Chang YH, Sieber M, Chiu TH, Ho M, Peng CT, Wu HP, Huang JL. Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation. 2011; 91:1412–1416. DOI: 10.1097/TP.0b013e31821aba18. PMID: 21494176.
Article37. Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974; 18:295–304. DOI: 10.1097/00007890-197410000-00001. PMID: 4153799.
Article38. Boruczkowski D, Gładysz D, Rumiński S, Czaplicka-Szmaus I, Murzyn M, Olkowicz A, Kałwak K, Mielcarek M, Drabko K, Styczyński J, Markiewicz M, Pawelec K, Boruczkowski M, Ołdak T. Third-party Wharton’s jelly mesenchymal stem cells for treatment of steroid-resistant acute and chronic graft-versus-host disease: a report of 10 cases. Turk J Biol. 2016; 40:493–500. DOI: 10.3906/biy-1508-47.
Article39. Wu QL, Liu XY, Nie DM, Zhu XX, Fang J, You Y, Zhong ZD, Xia LH, Hong M. Umbilical cord blood-derived mesenchymal stem cells ameliorate graft-versus-host disease following allogeneic hematopoietic stem cell transplantation through multiple immunoregulations. J Huazhong Univ Sci Technolog Med Sci. 2015; 35:477–484. DOI: 10.1007/s11596-015-1456-8. PMID: 26223913.
Article40. Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG, Yan XY, Wang Y, Zhu ZM, Li TC, Wang LH, Chen HY, Chen YD, Huang CL, Qu P, Yao C, Wang B, Chen GH, Wang ZM, Xu ZY, Bai J, Lu D, Shen YH, Guo F, Liu MY, Yang Y, Ding YC, Yang Y, Tian HT, Ding QA, Li LN, Yang XC, Hu X. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015; 13:162. DOI: 10.1186/s12916-015-0399-z. PMID: 26162993. PMCID: PMC4499169.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells
- A Case Of Intrauterine Fetal Death Due To Stricture Of The Umbilical Cord
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum
- Systemic aging delay and anti-aging therapy using allogeneic stem cells