Int J Stem Cells.
2014 Nov;7(2):118-126. 10.15283/ijsc.2014.7.2.118.
Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells
- Affiliations
-
- 1Department of Biology, Tabriz Branch, Islamic Azad University, Tabriz, Iran. Maleki.Masoud@gmail.com
- 2Stem Cell Research Lab, Azarbaijan ART Centre, ACECR East Azarbaijan Branch, Tabriz, Iran.
- KMID: 1974599
- DOI: http://doi.org/10.15283/ijsc.2014.7.2.118
Abstract
OBJECTIVES
Mesenchymal stem cells (MSCs) are adult stem cells which identified by adherence to plastic, expression of cell surface markers including CD44, CD90, CD105, CD106, CD166, and Stro-1, lack of the expression of hematopoietic markers, no immunogenic effect and replacement of damaged tissues. These properties led to development of progressive methods to isolation and characterization of MSCs from various sources for therapeutic applications in regenerative medicine.
METHODS
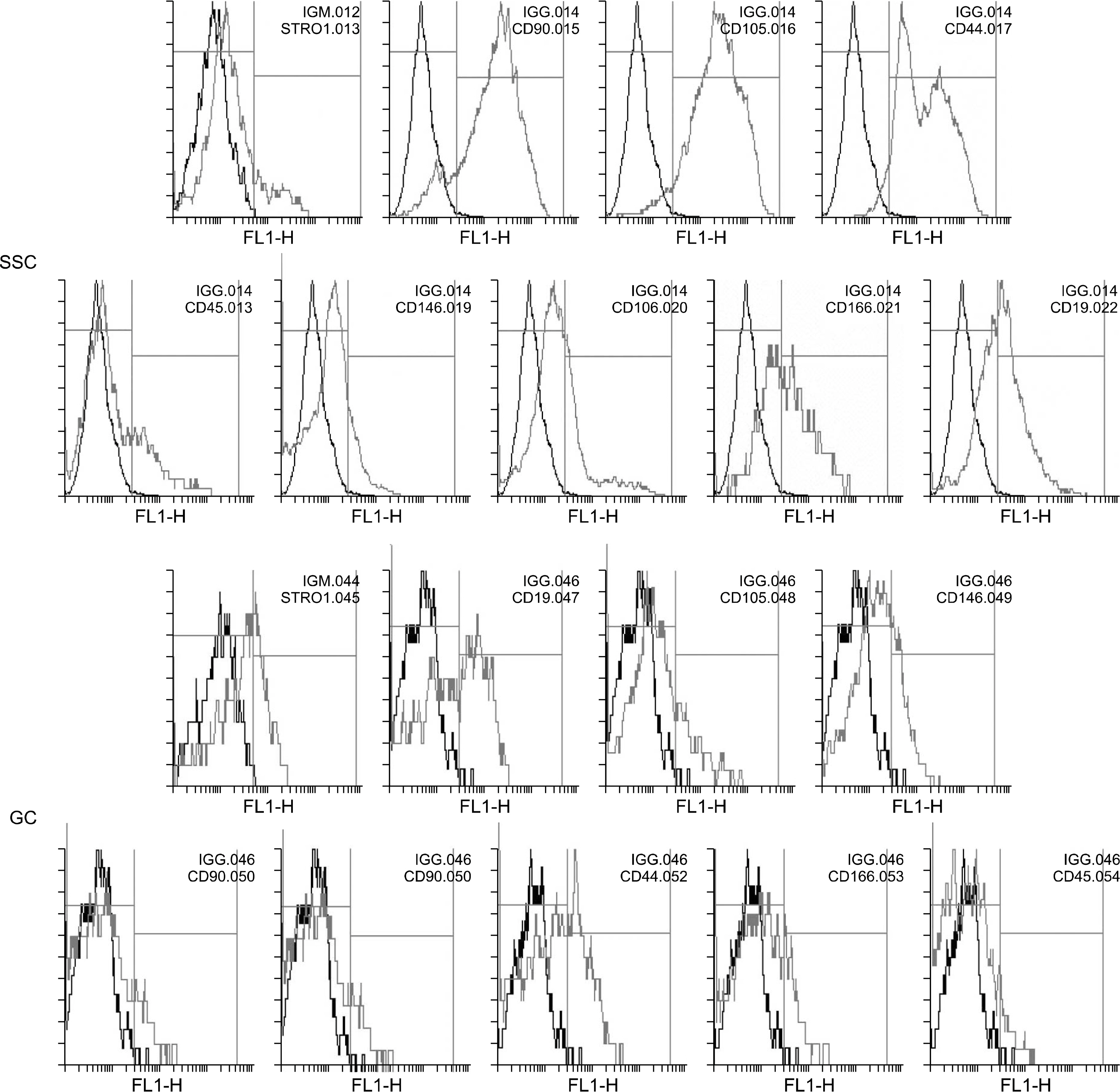
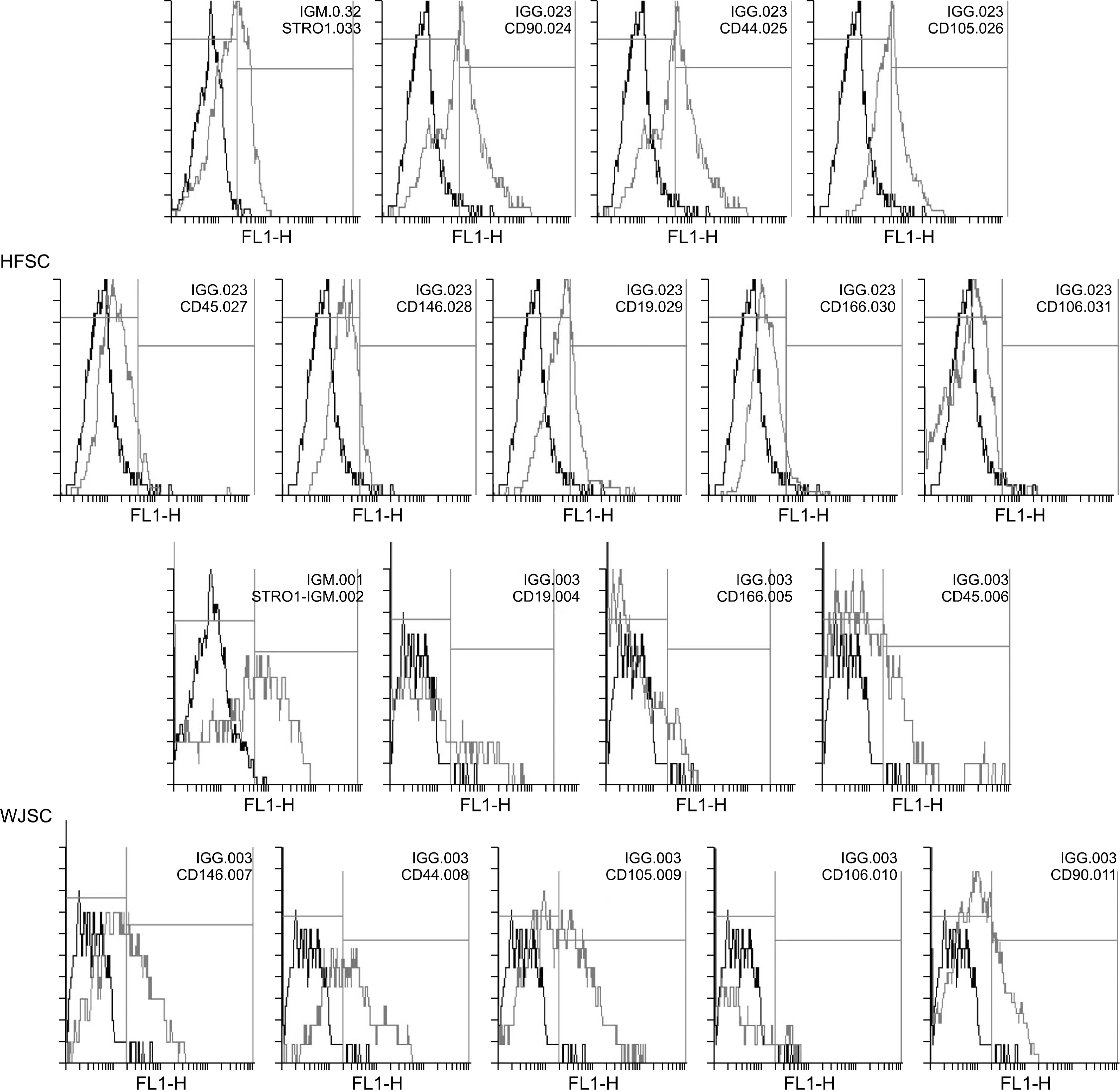
We isolated MSC-like cells from testis biopsies, ovary, hair follicle and umbilical cord Wharton's jelly and investigated the expression of specific cell surface antigens using flow cytometry in order to verify stemness properties of these cells.
RESULTS
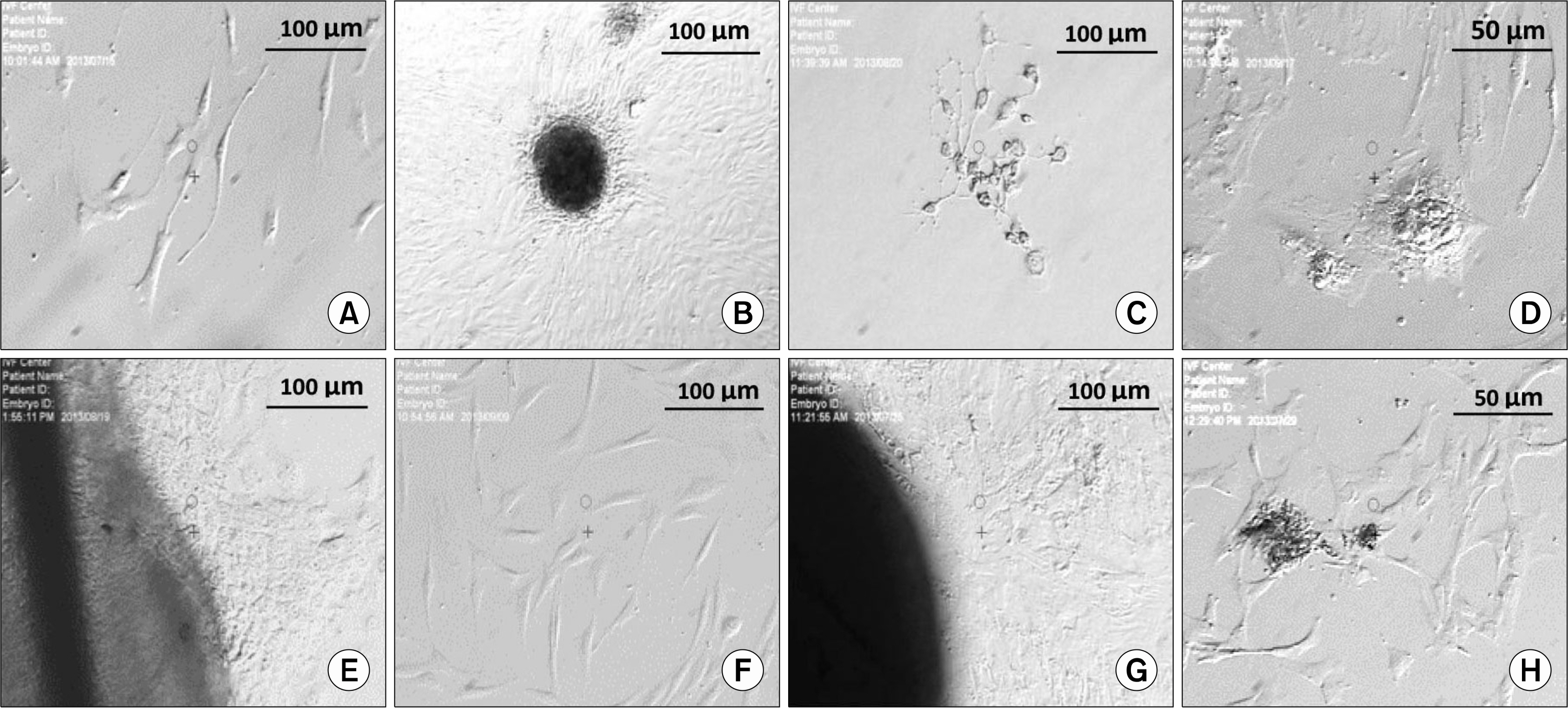
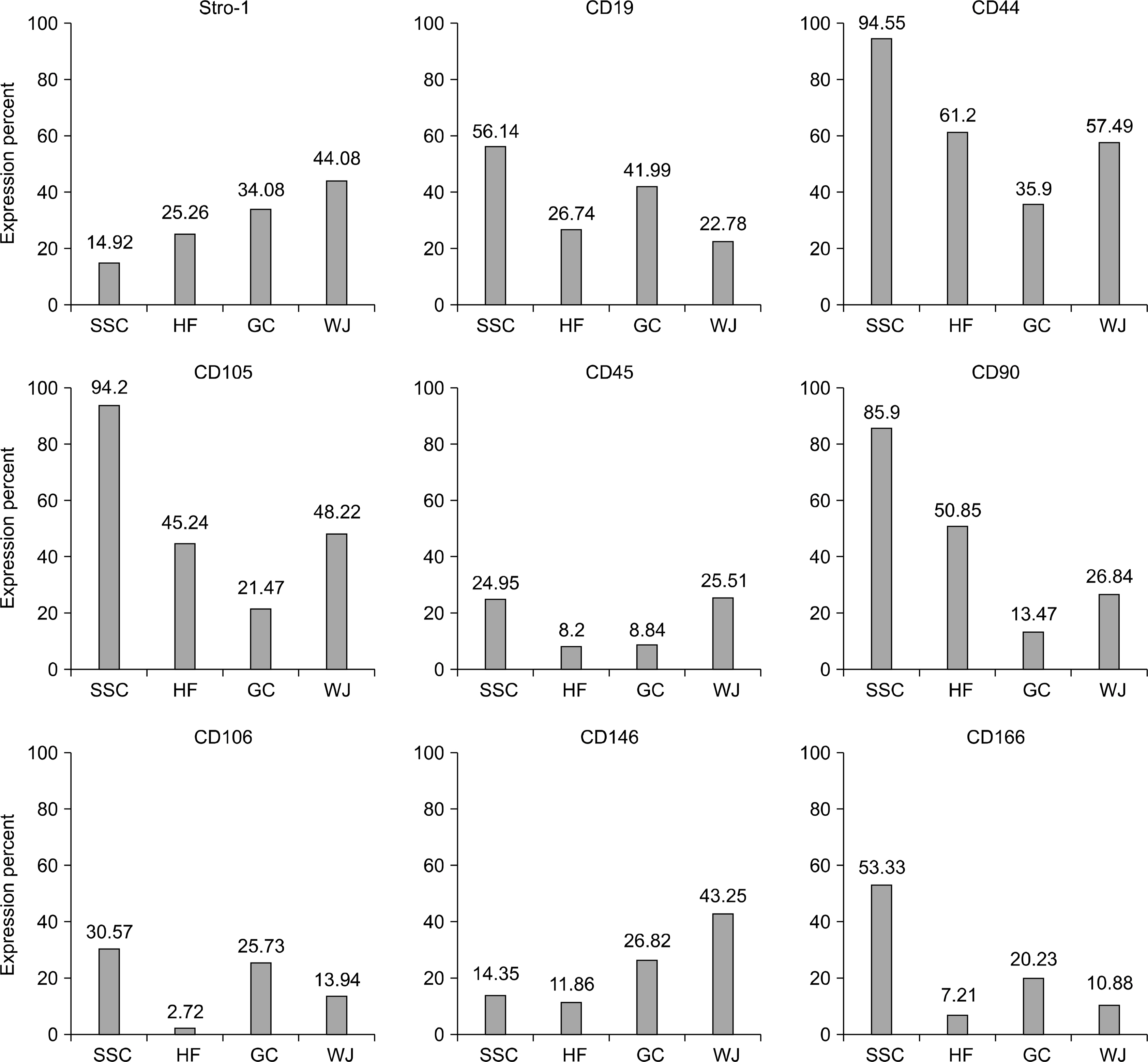
All four cell types adhered to plastic culture flask a few days after primary culture. All our cells positively expressed common MSC-specific cell surface markers. Moreover, our results revealed the expression of CD19and CD45 antigens in these cells.
CONCLUSION
According to our results, high expression of CD44 in spermatogonial stem cells (SSCs), hair follicle stem cells (HFSCs),granulosa cells (GCs)and Wharton's jelly-MSCs (WJ-MSCs)may help them to maintain stemness properties. Furthermore, we suggest that CD105+SSCs, HFSCs and WJ-MSCs revealed the osteogenic potential of these cells. Moreover, high expression of CD90 in SSCs and HFSCs may associate to higher growth and differentiation potential of these cells. Further, the presence of CD19 on SSCs and GCs may help them to efficiency in response to transmembrane signals. Thus, these four types of MSCs may be useful in clinical applications and cell therapy.
MeSH Terms
Figure
Cited by 1 articles
-
CD73, CD90, CD105 and Cadherin-11 RT-PCR Screening for Mesenchymal Stem Cells from Cryopreserved Human Cord Tissue
Hung Pham, Richard Tonai, Miya Wu, Chiara Birtolo, Monica Chen
Int J Stem Cells. 2018;11(1):26-38. doi: 10.15283/ijsc17015.
Reference
-
References
1. Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008. 132:598–611.
Article2. Avasthi S, Srivastava RN, Singh A, Srivastava M. Stem Cell: past, present and future: a review article. Internet Journal of Medical Update. 2008. 3:22–30.
Article3. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005. 52:2521–2529.
Article4. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.
Article5. Gonzalez R, Griparic L, Vargas V, Burgee K, Santacruz P, Anderson R, Schiewe M, Silva F, Patel A. A putative mesenchymal stem cells population isolated from adult human testes. Biochem Biophys Res Commun. 2009. 385:570–575.
Article6. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.
Article7. Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007. 293:L131–L141.
Article8. Helmy KY, Patel SA, Silverio K, Pliner L, Rameshwar P. Stem cells and regenerative medicine: accomplishments to date and future promise. Ther Deliv. 2010. 1:693–705.
Article9. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976. 4:267–274.10. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article11. Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006. 20:1045–1054.
Article12. Schieker M, Pautke C, Reitz K, Hemraj I, Neth P, Mutschler W, Milz S. The use of four-colour immunofluorescence techniques to identify mesenchymal stem cells. J Anat. 2004. 204:133–139.
Article13. Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006. 24:928–935.
Article14. Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002. 59:36–44.
Article15. Aslan H, Zilberman Y, Kandel L, Liebergall M, Oskouian RJ, Gazit D, Gazit Z. Osteogenic differentiation of non-cultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells. 2006. 24:1728–1737.
Article16. Roura S, Farré J, Soler-Botija C, Llach A, Hove-Madsen L, Cairó JJ, Gòdia F, Cinca J, Bayes-Genis A. Effect of aging on the pluripotential capacity of human CD105+ mesenchymal stem cells. Eur J Heart Fail. 2006. 8:555–563.
Article17. Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun. 1999. 265:134–139.
Article18. Pepinsky B, Hession C, Chen LL, Moy P, Burkly L, Jakubowski A, Chow EP, Benjamin C, Chi-Rosso G, Luhowskyj S, et al. Structure/function studies on vascular cell adhesion molecule-1. J Biol Chem. 1992. 267:17820–17826.
Article19. Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995. 121:489–503.
Article20. Kundrotas G. Surface markers distinguishing mesenchymal stem cells from fibroblasts. ActaMedica Lituanica. 2012. 19:75–79.
Article21. Ouhtit A, Gaur RL, Abd Elmageed ZY, Fernando A, Thouta R, Trappey AK, Abdraboh ME, El-Sayyad HI, Rao P, Raj MG. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim Biophys Acta. 2009. 1795:130–136.
Article22. Tremmel M, Matzke A, Albrecht I, Laib AM, Olaku V, Ballmer-Hofer K, Christofori G, Héroult M, Augustin HG, Ponta H, Orian-Rousseau V. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood. 2009. 114:5236–5244.
Article23. Kang Y, Wang F, Feng J, Yang D, Yang X, Yan X. Knockdown of CD146 reduces the migration and proliferation of human endothelial cells. Cell Res. 2006. 16:313–318.
Article24. Liu Q, Zhang B, Zhao X, Zhang Y, Liu Y, Yan X. Blockade of adhesion molecule CD146 causes pregnancy failure in mice. J Cell Physiol. 2008. 215:621–626.
Article25. Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999. 189:4–11.
Article26. Ohneda O, Ohneda K, Arai F, Lee J, Miyamoto T, Fukushima Y, Dowbenko D, Lasky LA, Suda T. ALCAM (CD166): its role in hematopoietic and endothelial development. Blood. 2001. 98:2134–2142.
Article27. Swart GW. Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol. 2002. 81:313–321.
Article28. Fujiwara H, Tatsumi K, Kosaka K, Sato Y, Higuchi T, Yoshioka S, Maeda M, Ueda M, Fujii S. Human blastocysts and endometrial epithelial cells express activated leukocyte cell adhesion molecule (ALCAM/CD166). J Clin Endocrinol Metab. 2003. 88:3437–3443.
Article29. Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991. 78:55–62.
Article30. Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, Madon E, Fagioli F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006. 97:744–754.
Article31. Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are down-regulated with passaging. Stem Cells Dev. 2011. 20:53–66.
Article32. Bensidhoum M, Chapel A, Francois S, Demarquay C, Mazurier C, Fouillard L, Bouchet S, Bertho JM, Gourmelon P, Aigueperse J, Charbord P, Gorin NC, Thierry D, Lopez M. Homing of in vitro expanded Stro-1− or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 2004. 103:3313–3319.
Article33. Carter RH, Barrington RA. Signaling by the CD19/CD21 complex on B cells. Curr Dir Autoimmun. 2004. 7:4–32.
Article34. Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol . 2006. 7(Suppl 1):S12.1–14.35. Wang K, Wei G, Liu D. CD19: a biomarker for B cell development,lymphoma diagnosis and therapy. Experimental Hematology and Oncology. 2012. 1:1–7.36. Poe JC, Minard-Colin V, Kountikov EI, Haas KM, Tedder TF. A c-Myc and surface CD19 signaling amplification loop promotes B cell lymphoma development and progression in mice. J Immunol. 2012. 189:2318–2325.
Article37. Shivtiel S, Kollet O, Lapid K, Schajnovitz A, Goichberg P, Kalinkovich A, Shezen E, Tesio M, Netzer N, Petit I, Sharir A, Lapidot T. CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J Exp Med. 2008. 205:2381–2395.
Article38. Wang Y, Liu J, Tan X, Li G, Gao Y, Liu X, Zhang L, Li Y. Induced pluripotent stem cells from human hair follicle mesenchymal stem cells. Stem Cell Rev. 2013. 9:451–460.
Article39. Kossowska-Tomaszczuk K, De Geyter C, De Geyter M, Martin I, Holzgreve W, Scherberich A, Zhang H. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells. 2009. 27:210–219.
Article40. Gatta V, D’Aurora M, Lanuti P, Pierdomenico L, Sperduti S, Palka G, Gesi M, Marchisio M, Miscia S, Stuppia L. Gene expression modifications in Wharton’s Jelly mesenchymal stem cells promoted by prolonged in vitro culturing. BMC Genomics. 2013. 14:635.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- The hope and hype of stem cell therapy
- Cell Biological Characteristics of Adult Stem Cells