Yonsei Med J.
2016 May;57(3):761-768. 10.3349/ymj.2016.57.3.761.
STAT3 and ERK Signaling Pathways Are Implicated in the Invasion Activity by Oncostatin M through Induction of Matrix Metalloproteinases 2 and 9
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Seoul, Korea. jcshin@catholic.ac.kr
- 2Department of Biochemistry, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2374099
- DOI: http://doi.org/10.3349/ymj.2016.57.3.761
Abstract
- PURPOSE
Our previous studies have shown that oncostatin M (OSM) promotes trophoblast invasion activity through increased enzyme activity of matrix metalloproteinase (MMP)-2 and -9. We further investigated OSM-induced intracellular signaling mechanisms associated with these events in the immortalized human trophoblast cell line HTR8/SVneo.
MATERIALS AND METHODS
We investigated the effects of OSM on RNA and protein expression of MMP-2 and -9 in the first-trimester extravillous trophoblast cell line (HTR8/SVneo) via Western blot. The selective signal transducer and activator of transcription (STAT)3 inhibitor, stattic, STAT3 siRNA, and extracellular signal-regulated kinase (ERK) siRNA were used to investigate STAT3 and ERK activation by OSM. The effects of STAT3 and ERK inhibitors on OSM-induced enzymatic activities of MMP-2 and -9 and invasion activity were further determined via Western blot and gelatin zymography.
RESULTS
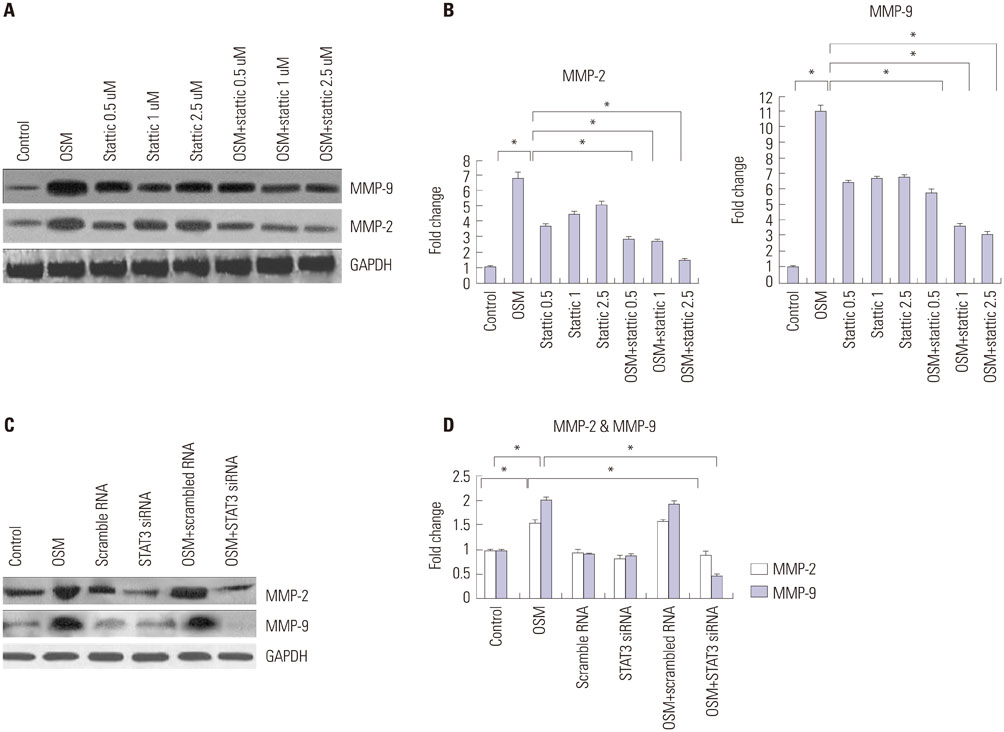
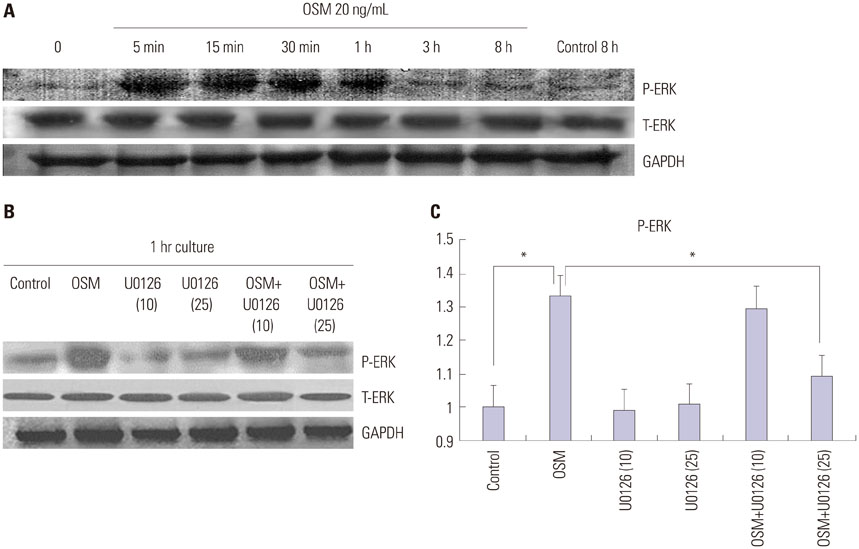
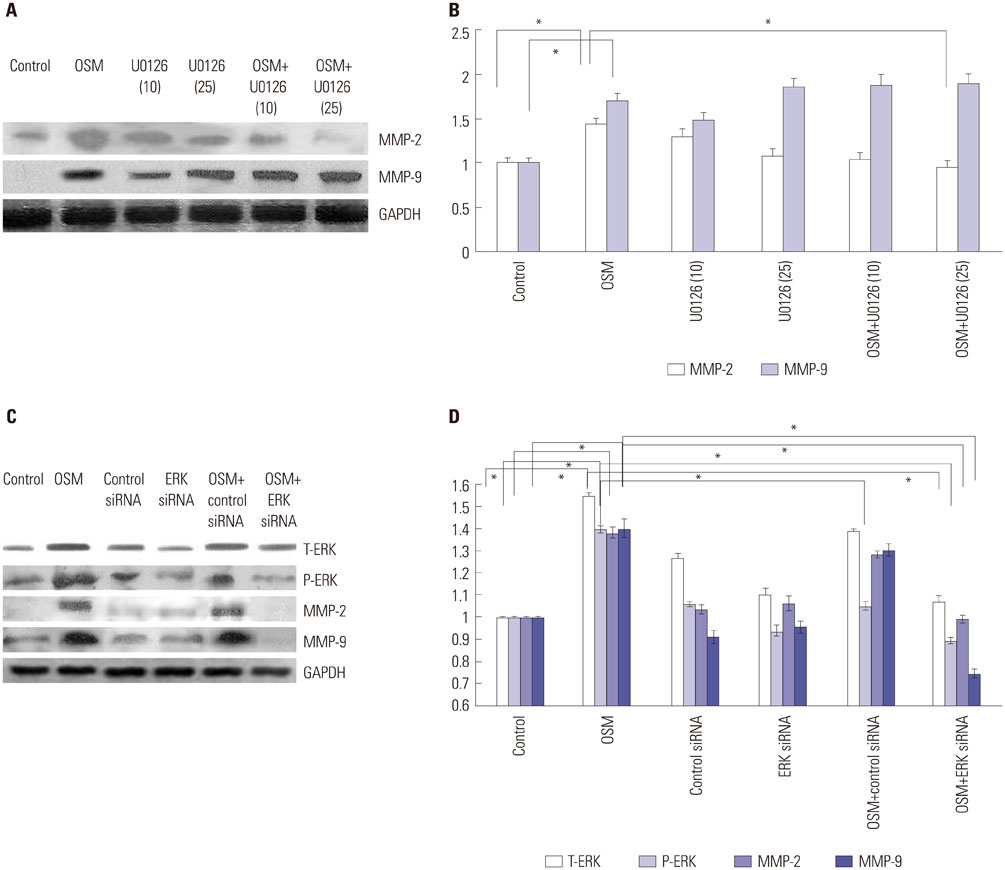
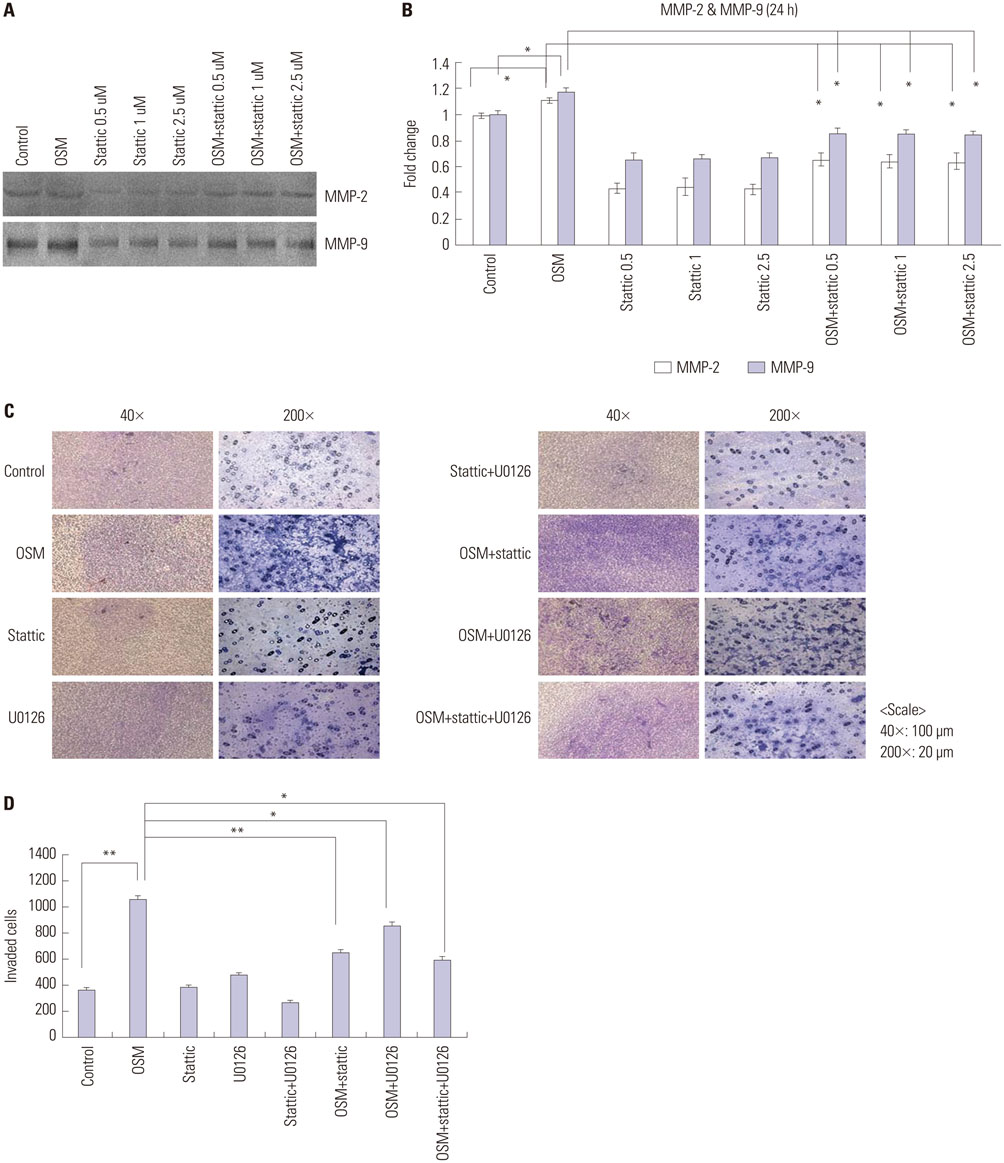
OSM-induced MMP-2 and -9 protein expression was significantly suppressed by STAT3 inhibition with stattic and STAT3 siRNA silencing, whereas the ERK1/2 inhibitor (U0126) and ERK silencing significantly suppressed OSM-induced MMP-2 protein expression. OSM-induced MMP-2 and MMP-9 enzymatic activities were significantly decreased by stattic pretreatment. The increased invasion activity induced by OSM was significantly suppressed by STAT3 and ERK1/2 inhibition, though to a greater extent by STAT3 inhibition.
CONCLUSION
Both STAT3 and ERK signaling pathways are involved in OSM-induced invasion activity of HTR8/SVneo cells. Activation of STAT3 appears to be critical for the OSM-mediated increase in invasiveness of HTR8/SVneo cells.
Keyword
MeSH Terms
-
Blotting, Western
Cell Movement/*drug effects
Cell Proliferation/*drug effects
Extracellular Signal-Regulated MAP Kinases/metabolism
Humans
Matrix Metalloproteinase 2/genetics/*metabolism
Matrix Metalloproteinase 9/genetics/*metabolism
Oncostatin M/genetics/*metabolism
Phosphorylation/drug effects
RNA, Messenger/metabolism
RNA, Small Interfering
STAT3 Transcription Factor/*metabolism
Signal Transduction/*drug effects
Extracellular Signal-Regulated MAP Kinases
Matrix Metalloproteinase 2
Matrix Metalloproteinase 9
Oncostatin M
RNA, Messenger
RNA, Small Interfering
STAT3 Transcription Factor
Figure
Cited by 1 articles
-
Effects of Oncostatin M on Invasion of Primary Trophoblasts under Normoxia and Hypoxia Conditions
Jeong ha Wie, Hyun Sun Ko, Sae Kyung Choi, In Yang Park, Ahyoung Kim, Ho Shik Kim, Jong Chul Shin
Yonsei Med J. 2018;59(7):879-886. doi: 10.3349/ymj.2018.59.7.879.
Reference
-
1. Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006; 27:783–793.
Article2. Ogata I, Shimoya K, Moriyama A, Shiki Y, Matsumura Y, Yamanaka K, et al. Oncostatin M is produced during pregnancy by decidual cells and stimulates the release of HCG. Mol Hum Reprod. 2000; 6:750–757.
Article3. Fitzgerald JS, Tsareva SA, Poehlmann TG, Berod L, Meissner A, Corvinus FM, et al. Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int J Biochem Cell Biol. 2005; 37:2284–2296.
Article4. Ko HS, Kang HK, Kim HS, Choi SK, Park IY, Shin JC. The effects of oncostatin M on trophoblast cells: influence on matrix metalloproteinases-2 and -9, and invasion activity. Placenta. 2012; 33:908–913.
Article5. Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993; 206:204–211.
Article6. Irving JA, Lysiak JJ, Graham CH, Hearn S, Han VK, Lala PK. Characteristics of trophoblast cells migrating from first trimester chorionic villus explants and propagated in culture. Placenta. 1995; 16:413–433.
Article7. Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod. 2000; 62:739–747.
Article8. Busch S, Renaud SJ, Schleussner E, Graham CH, Markert UR. mTOR mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of STAT3. Exp Cell Res. 2009; 315:1724–1733.
Article9. Ko HS, Choi SK, Kang HK, Kim HS, Jeon JH, Park IY, et al. Oncostatin M stimulates cell migration and proliferation by down-regulating E-cadherin in HTR8/SVneo cell line through STAT3 activation. Reprod Biol Endocrinol. 2013; 11:93.
Article10. Poehlmann TG, Fitzgerald JS, Meissner A, Wengenmayer T, Schleussner E, Friedrich K, et al. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta. 2005; 26:Suppl A. S37–S41.
Article11. Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002; 8:945–954.12. Suman P, Malhotra SS, Gupta SK. LIF-STAT signaling and trophoblast biology. JAKSTAT. 2013; 2:e25155.
Article13. Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR. Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3). Hum Reprod Update. 2008; 14:335–344.
Article14. Fossey SL, Bear MD, Kisseberth WC, Pennell M, London CA. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer. 2011; 11:125.
Article15. Snyder M, Huang XY, Zhang JJ. Signal transducers and activators of transcription 3 (STAT3) directly regulates cytokine-induced fascin expression and is required for breast cancer cell migration. J Biol Chem. 2011; 286:38886–38893.
Article16. Pijnenborg R. Implantation and immunology: maternal inflammatory and immune cellular responses to implantation and trophoblast invasion. Reprod Biomed Online. 2002; 4:Suppl 3. 14–17.
Article17. Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997; 277:1669–1672.
Article18. De Marco CS, Caniggia I. Mechanisms of oxygen sensing in human trophoblast cells. Placenta. 2002; 23:Suppl A. S58–S68.
Article19. Zygmunt M, Hahn D, Münstedt K, Bischof P, Lang U. Invasion of cytotrophoblastic JEG-3 cells is stimulated by hCG in vitro. Placenta. 1998; 19:587–593.
Article20. Mandl M, Haas J, Bischof P, Nöhammer G, Desoye G. Serum-dependent effects of IGF-I and insulin on proliferation and invasion of human first trimester trophoblast cell models. Histochem Cell Biol. 2002; 117:391–399.
Article21. Liu HY, Jia XQ, Gao LX, Ma YY. Hepatocyte growth factor regulates HLX1 gene expression to modulate HTR-8/SVneo trophoblast cells. Reprod Biol Endocrinol. 2012; 10:83.
Article22. Cartwright JE, Holden DP, Whitley GS. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol. 1999; 128:181–189.
Article23. Lash GE, Cartwright JE, Whitley GS, Trew AJ, Baker PN. The effects of angiogenic growth factors on extravillous trophoblast invasion and motility. Placenta. 1999; 20:661–667.
Article24. Bilban M, Tauber S, Haslinger P, Pollheimer J, Saleh L, Pehamberger H, et al. Trophoblast invasion: assessment of cellular models using gene expression signatures. Placenta. 2010; 31:989–996.
Article25. Lash GE, Hornbuckle J, Brunt A, Kirkley M, Searle RF, Robson SC, et al. Effect of low oxygen concentrations on trophoblast-like cell line invasion. Placenta. 2007; 28:390–398.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Oncostatin M on Invasion of Primary Trophoblasts under Normoxia and Hypoxia Conditions
- Extracellular Signal-regulated Kinase Activation Is Required for Serine 727 Phosphorylation of STAT3 in Schwann Cells in vitro and in vivo
- Inhibition of the Interleukin-11-STAT3 Axis Attenuates Hypoxia-Induced Migration and Invasion in MDA-MB-231 Breast Cancer Cells
- Suppression of CD81 promotes bladder cancer cell invasion through increased matrix metalloproteinase expression via extracellular signal-regulated kinase phosphorylation
- Roles of Matrix Metalloproteinases and Their Targets in Epileptogenesis and Seizures