Yonsei Med J.
2018 Sep;59(7):879-886. 10.3349/ymj.2018.59.7.879.
Effects of Oncostatin M on Invasion of Primary Trophoblasts under Normoxia and Hypoxia Conditions
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Seoul, Korea. jcshin@catholic.ac.kr
- 2Department of Biochemistry, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2428915
- DOI: http://doi.org/10.3349/ymj.2018.59.7.879
Abstract
- PURPOSE
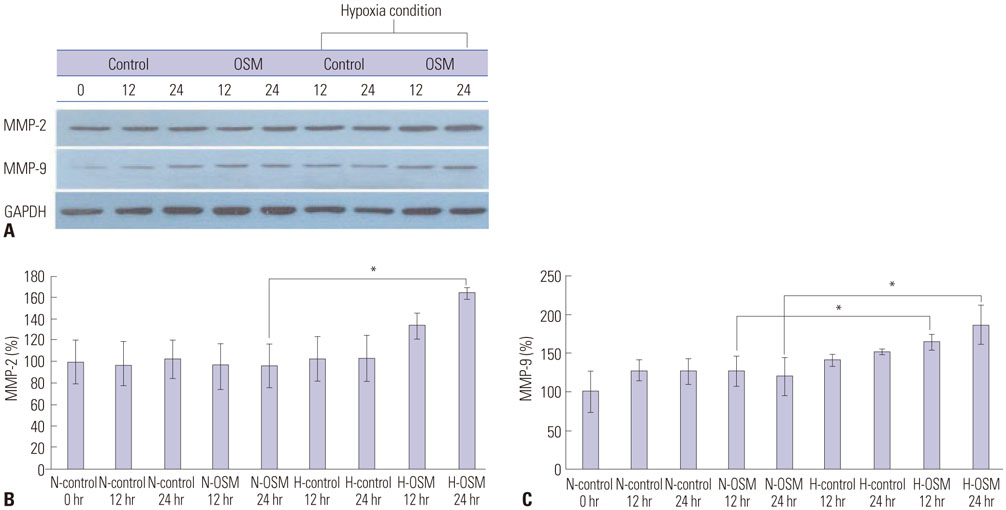
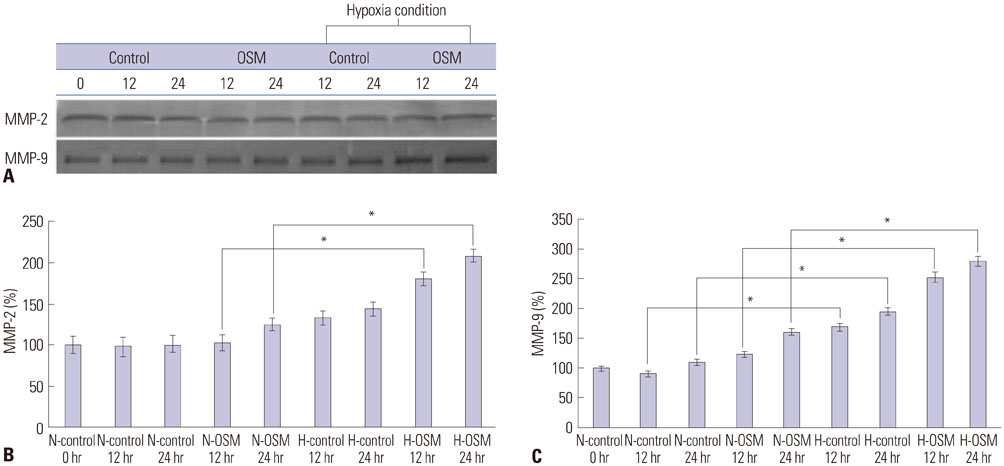
To investigate the effect of oncostatin M (OSM) on protein expression levels and enzymatic activities of matrix metalloprotainase (MMP)-2 and MMP-9 in primary trophoblasts and the invasiveness thereof under normoxia and hypoxia conditions.
MATERIALS AND METHODS
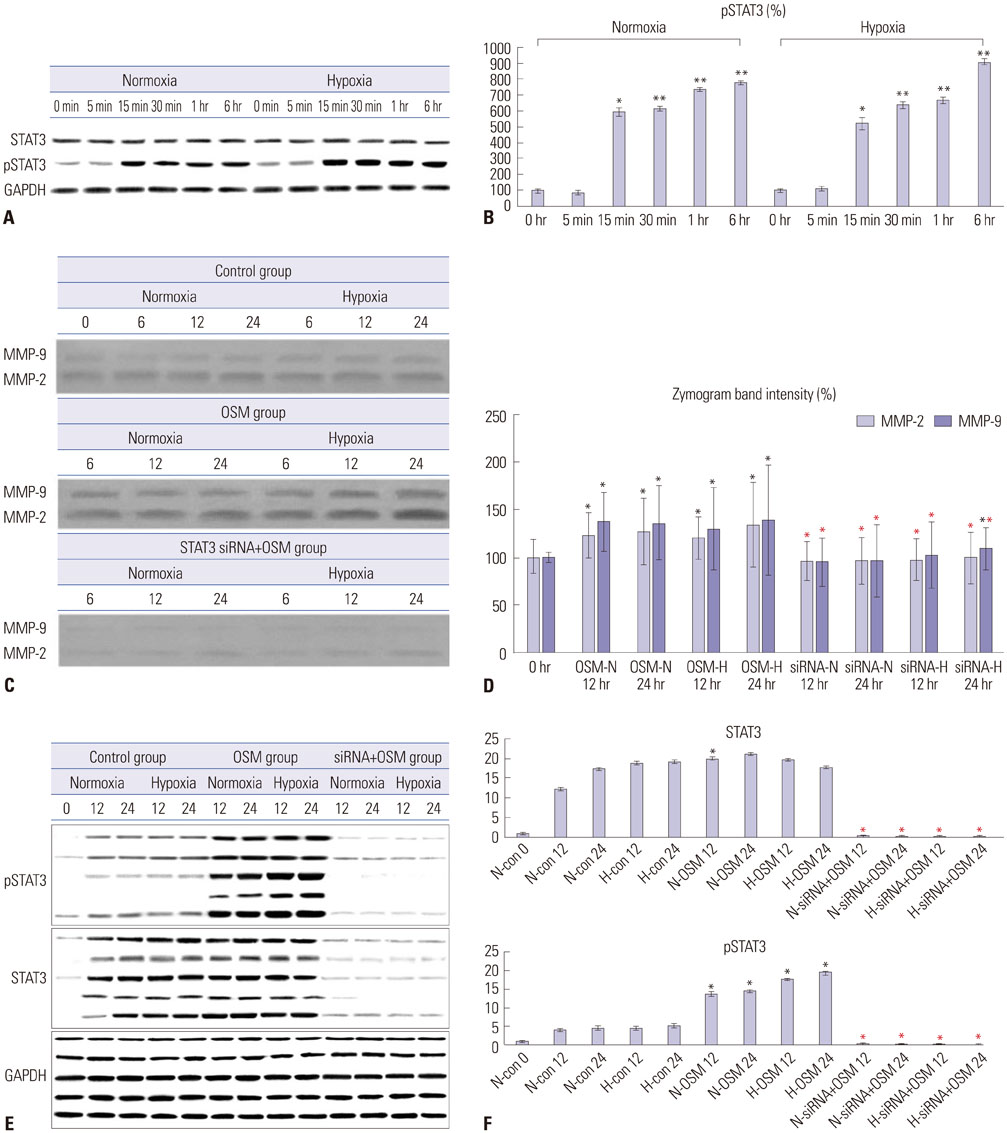
Protein expression levels and enzymatic activities of MMP-2 and MMP-9 in primary trophoblasts under normoxia and hypoxia conditions were examined by Western blot and zymography, respectively. Effects of exogenous OSM on the in vitro invasion activity of trophoblasts according to oxygen concentration were also determined. Signal transducer and activator of transcription 3 (STAT3) siRNA was used to determine whether STAT3 activation in primary trophoblasts was involved in the effect of OSM.
RESULTS
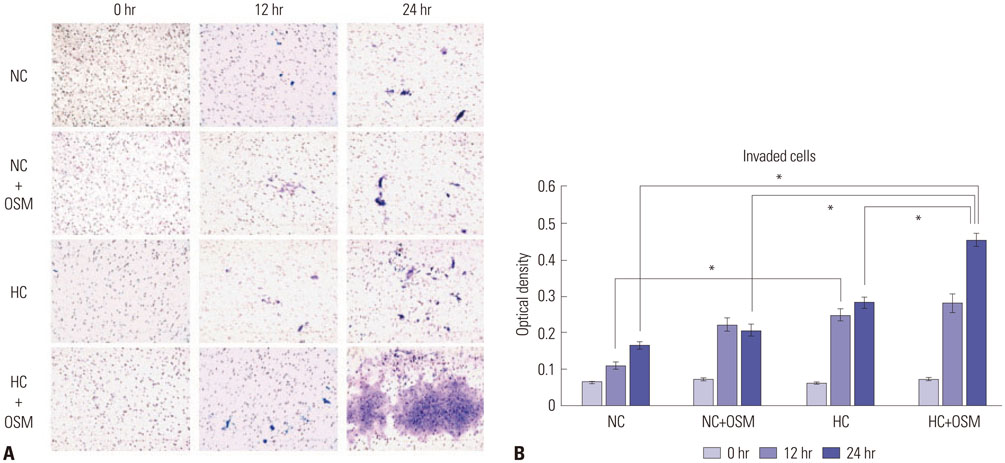
OSM enhanced protein expression levels and enzymatic activities of MMP-2 and MMP-9 in term trophoblasts under hypoxia condition, compared to normoxia control (p < 0.05). OSM-induced MMP-2 and MMP-9 enzymatic activities were significantly suppressed by STAT3 siRNA silencing under normoxia and hypoxia conditions (p < 0.05). Hypoxia alone or OSM alone did not significantly increase the invasiveness of term trophoblasts. However, the invasion activity of term trophoblasts was significantly increased by OSM under hypoxia, compared to that without OSM treatment under normoxia.
CONCLUSION
OSM might be involved in the invasiveness of extravillous trophoblasts under hypoxia conditions via increasing MMP-2 and MMP-9 enzymatic activities through STAT3 signaling. Increased MMP-9 activity by OSM seems to be more important in primary trophoblasts.
MeSH Terms
Figure
Reference
-
1. Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR. Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3). Hum Reprod Update. 2008; 14:335–344.
Article2. Loke YW, King A, Burrows TD. Decidua in human implantation. Hum Reprod. 1995; 10:Suppl 2. 14–21.
Article3. Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994; 101:669–674.
Article4. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003; 69:1–7.
Article5. Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008; 314:362–375.
Article6. Soares MJ, Chakraborty D, Renaud SJ, Kubota K, Bu P, Konno T, et al. Regulatory pathways controlling the endovascular invasive trophoblast cell lineage. J Reprod Dev. 2012; 58:283–287.
Article7. Huppertz B, Weiss G, Moser G. Trophoblast invasion and oxygenation of the placenta: measurements versus presumptions. J Reprod Immunol. 2014; 101-102:74–79.
Article8. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993; 4:197–250.
Article9. Barsoum IB, Renaud SJ, Graham CH. Glyceryl trinitrate inhibits hypoxia-induced release of soluble fms-like tyrosine kinase-1 and endoglin from placental tissues. Am J Pathol. 2011; 178:2888–2896.
Article10. Tanaka M, Miyajima A. Oncostatin M, a multifunctional cytokine. Rev Physiol Biochem Pharmacol. 2003; 149:39–52.11. Ogata I, Shimoya K, Moriyama A, Shiki Y, Matsumura Y, Yamanaka K, et al. Oncostatin M is produced during pregnancy by decidual cells and stimulates the release of HCG. Mol Hum Reprod. 2000; 6:750–757.
Article12. Ko HS, Kang HK, Kim HS, Choi SK, Park IY, Shin JC. The effects of oncostatin M on trophoblast cells: influence on matrix metalloproteinases-2 and -9, and invasion activity. Placenta. 2012; 33:908–913.
Article13. Ko HS, Choi SK, Kang HK, Kim HS, Jeon JH, Park IY, et al. Oncostatin M stimulates cell migration and proliferation by down-regulating E-cadherin in HTR8/SVneo cell line through STAT3 activation. Reprod Biol Endocrinol. 2013; 11:93.
Article14. Ko HS, Park BJ, Choi SK, Kang HK, Kim A, Kim HS, et al. STAT3 and ERK signaling pathways are implicated in the invasion activity by oncostatin M through induction of matrix metalloproteinases 2 and 9. Yonsei Med J. 2016; 57:761–768.
Article15. Stenqvist AC, Chen T, Hedlund M, Dimova T, Nagaeva O, Kjellberg L, et al. An efficient optimized method for isolation of villous trophoblast cells from human early pregnancy placenta suitable for functional and molecular studies. Am J Reprod Immunol. 2008; 60:33–42.
Article16. Tarrade A, Lai Kuen R, Malassiné A, Tricottet V, Blain P, Vidaud M, et al. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest. 2001; 81:1199–1211.
Article17. Borbely AU, Sandri S, Fernandes IR, Prado KM, Cardoso EC, Correa-Silva S, et al. The term basal plate of the human placenta as a source of functional extravillous trophoblast cells. Reprod Biol Endocrinol. 2014; 12:7.
Article18. Ji YQ, Zhang YQ, Li MQ, Du MR, Wei WW, Li DJ. EPO improves the proliferation and inhibits apoptosis of trophoblast and decidual stromal cells through activating STAT-5 and inactivating p38 signal in human early pregnancy. Int J Clin Exp Pathol. 2011; 4:765–774.19. Awad AE, Kandalam V, Chakrabarti S, Wang X, Penninger JM, Davidge ST, et al. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kgamma-dependent manner. Am J Physiol Cell Physiol. 2010; 298:C679–C692.20. Poehlmann TG, Fitzgerald JS, Meissner A, Wengenmayer T, Schleussner E, Friedrich K, et al. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta. 2005; 26:Suppl A. S37–S41.
Article21. James JL, Stone PR, Chamley LW. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum Reprod Update. 2006; 12:137–144.
Article22. Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000; 21:Suppl A. S25–S30.
Article23. Knöfler M, Simmons DG, Lash GE, Harris LK, Armant DR. Regulation of trophoblast invasion-a workshop report. Placenta. 2008; 29:Suppl A. S26–S28.24. Bischof P, Meisser A, Campana A. Paracrine and autocrine regulators of trophoblast invasion--a review. Placenta. 2000; 21:Suppl A. S55–S60.25. Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997; 277:1669–1672.
Article26. Huppertz B, Gauster M, Orendi K, König J, Moser G. Oxygen as modulator of trophoblast invasion. J Anat. 2009; 215:14–20.
Article27. Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014; 10:466–480.
Article28. Graham CH, Fitzpatrick TE, McCrae KR. Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood. 1998; 91:3300–3307.
Article29. Lash GE, Hornbuckle J, Brunt A, Kirkley M, Searle RF, Robson SC, et al. Effect of low oxygen concentrations on trophoblast-like cell line invasion. Placenta. 2007; 28:390–398.
Article30. Luo J, Qiao F, Yin X. Hypoxia induces FGF2 production by vascular endothelial cells and alters MMP9 and TIMP1 expression in extravillous trophoblasts and their invasiveness in a cocultured model. J Reprod Dev. 2011; 57:84–91.
Article31. Huppertz B, Kingdom JC. Apoptosis in the trophoblast--role of apoptosis in placental morphogenesis. J Soc Gynecol Investig. 2004; 11:353–362.
Article32. Lee G, Kil G, Kwon J, Kim S, Yoo J, Shin J. Oncostatin M as a target biological molecule of preeclampsia. J Obstet Gynaecol Res. 2009; 869–875.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Controlled Gene Expression System under Hypoxia Conditions
- Effects of hypoxia inducible factors-1alpha on autophagy and invasion of trophoblasts
- Effects of selenium on the survival and invasion of trophoblasts
- ACY-241, a histone deacetylase 6 inhibitor, suppresses the epithelial–mesenchymal transition in lung cancer cells by downregulating hypoxia-inducible factor-1 alpha
- The Effect of Nitric Oxide on Cat Corpus Cavernosum Relaxation Under Hypoxia (In vivo Study)