Cancer Res Treat.
2017 Jan;49(1):79-91. 10.4143/crt.2015.503.
Anti-proliferative Effect of Engineered Neural Stem Cells Expressing Cytosine Deaminase and Interferon-β against Lymph Node–Derived Metastatic Colorectal Adenocarcinoma in Cellular and Xenograft Mouse Models
- Affiliations
-
- 1Laboratory of Biochemistry and Immunology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. kchoi@cbu.ac.kr
- 2Department of Medicine, Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada.
- 3TheraCell Bio & Science, Cheongju, Korea.
- KMID: 2367506
- DOI: http://doi.org/10.4143/crt.2015.503
Abstract
- PURPOSE
Genetically engineered stem cells may be advantageous for gene therapy against various human cancers due to their inherent tumor-tropic properties. In this study, genetically engineered human neural stem cells (HB1.F3) expressing Escherichia coli cytosine deaminase (CD) (HB1.F3.CD) and human interferon-β (IFN-β) (HB1.F3.CD.IFN-β) were employed against lymph node-derived metastatic colorectal adenocarcinoma.
MATERIALS AND METHODS
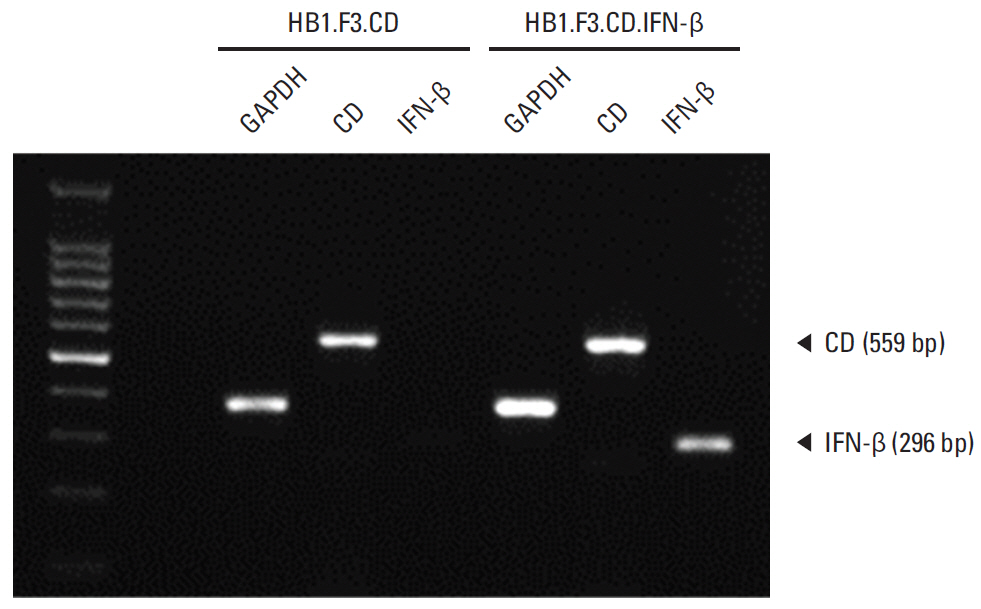
CD can convert a prodrug, 5-fluorocytosine (5-FC), to active 5-fluorouracil, which inhibits tumor growth through the inhibition of DNA synthesis,while IFN-β also strongly inhibits tumor growth by inducing the apoptotic process. In reverse transcription polymerase chain reaction analysis, we confirmed that HB1.F3.CD cells expressed the CD gene and HB1.F3.CD.IFN-β cells expressed both CD and IFN-β genes.
RESULTS
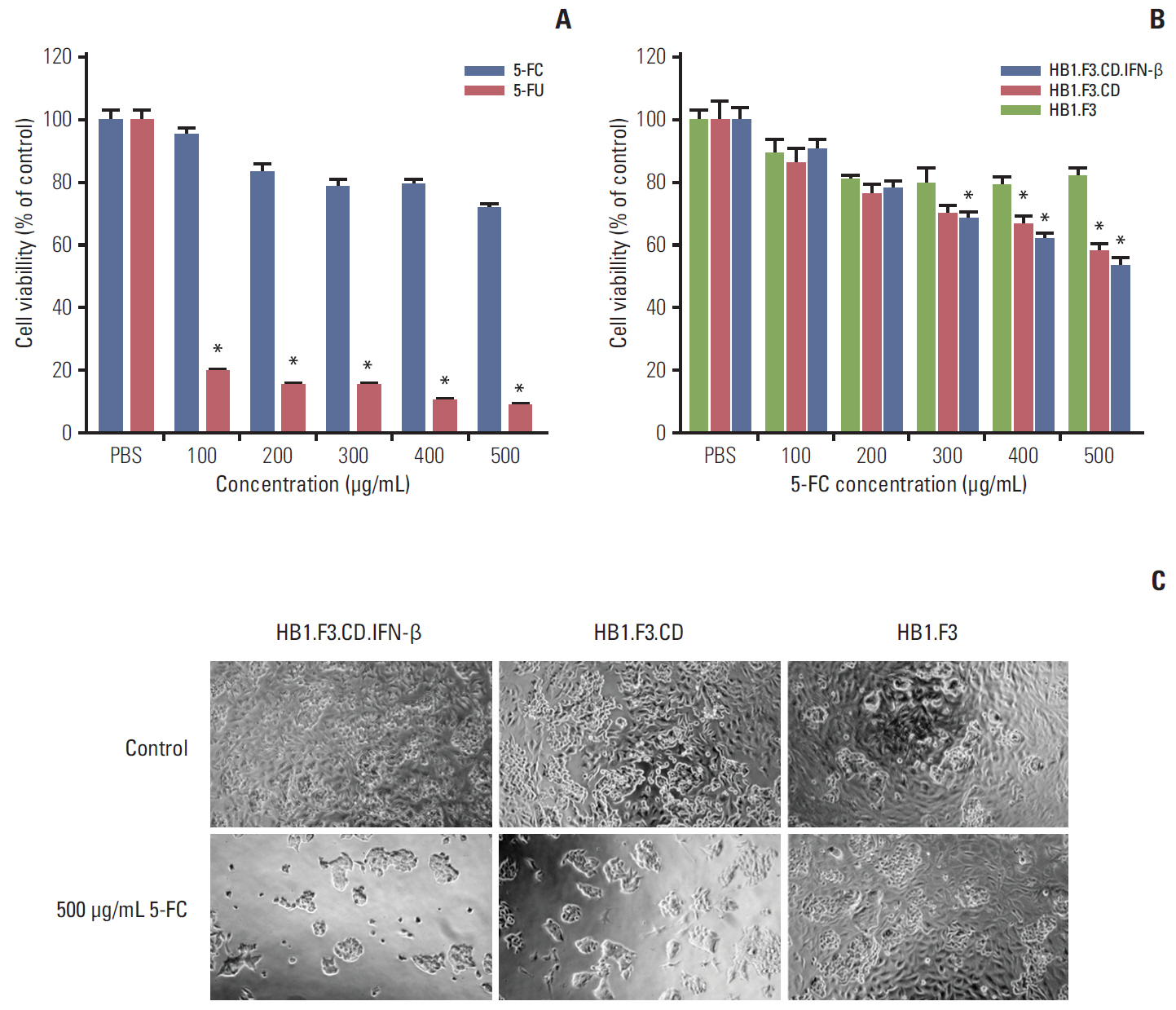
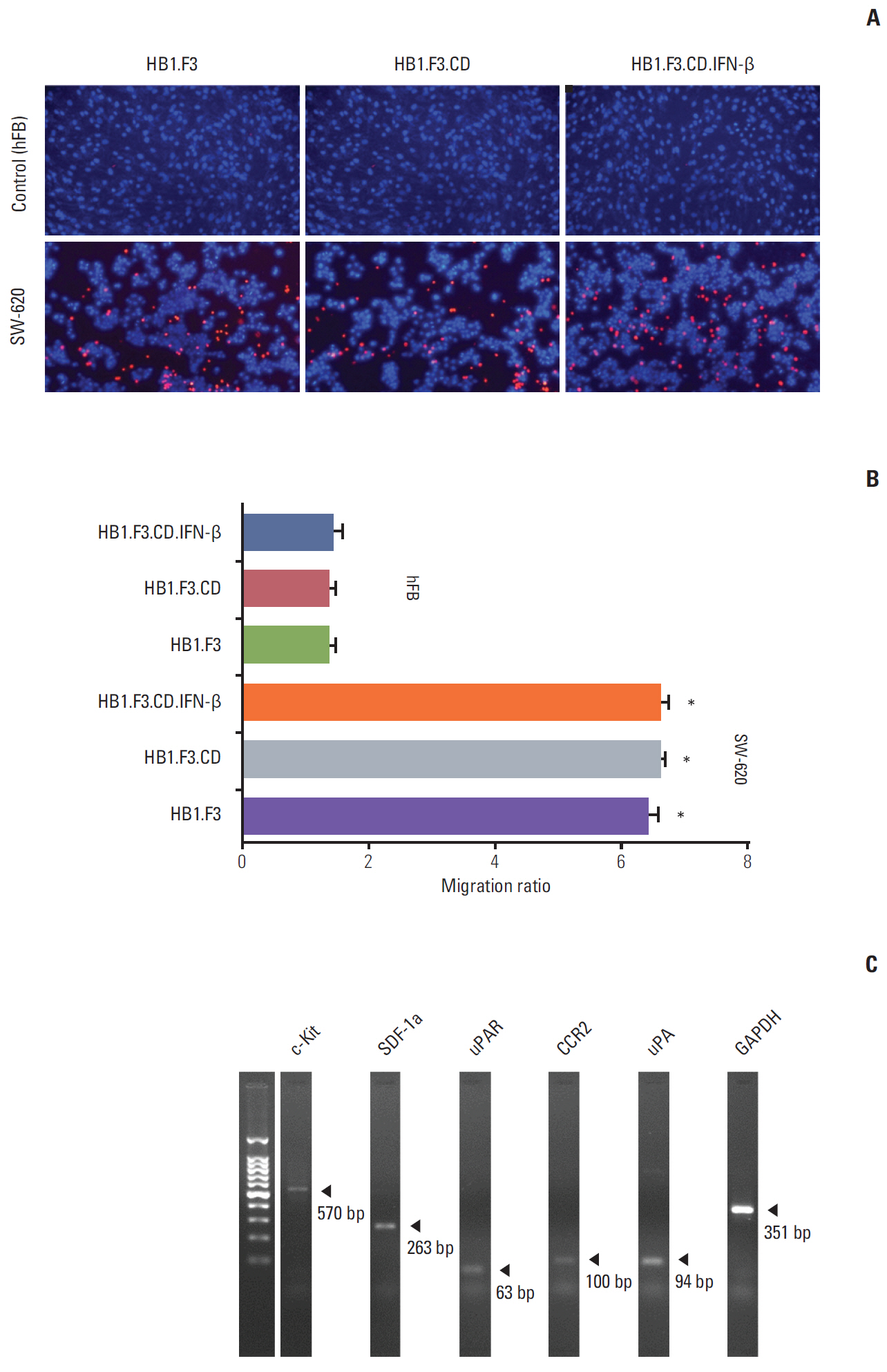
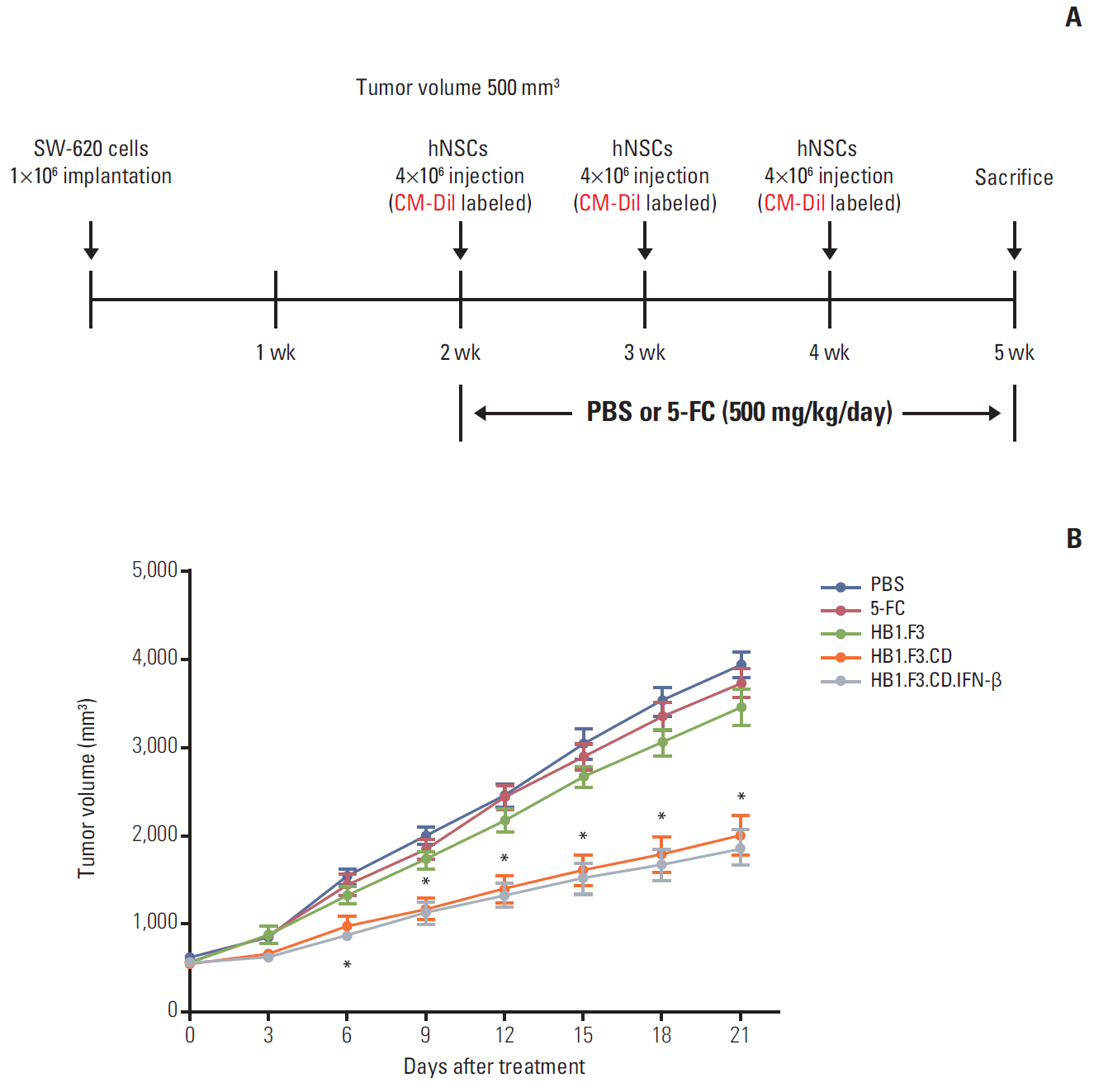
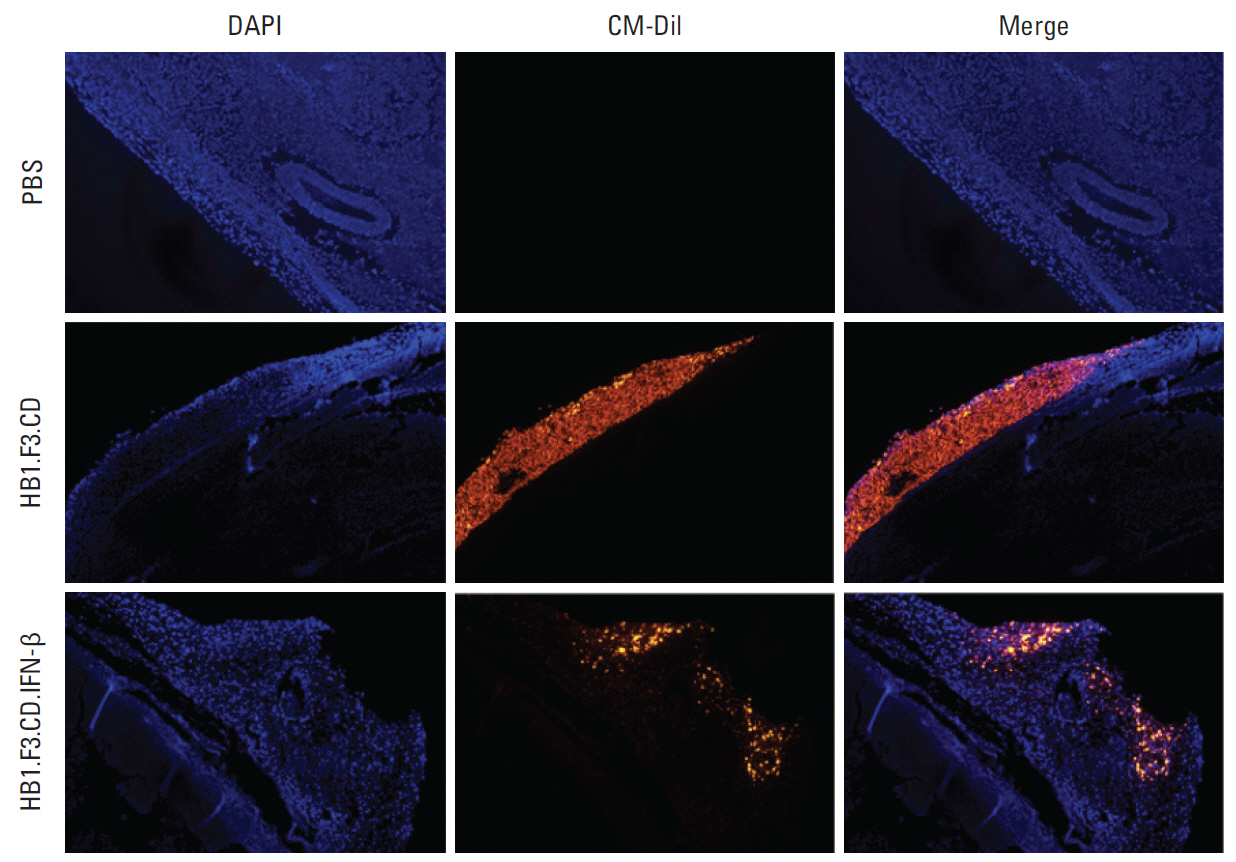
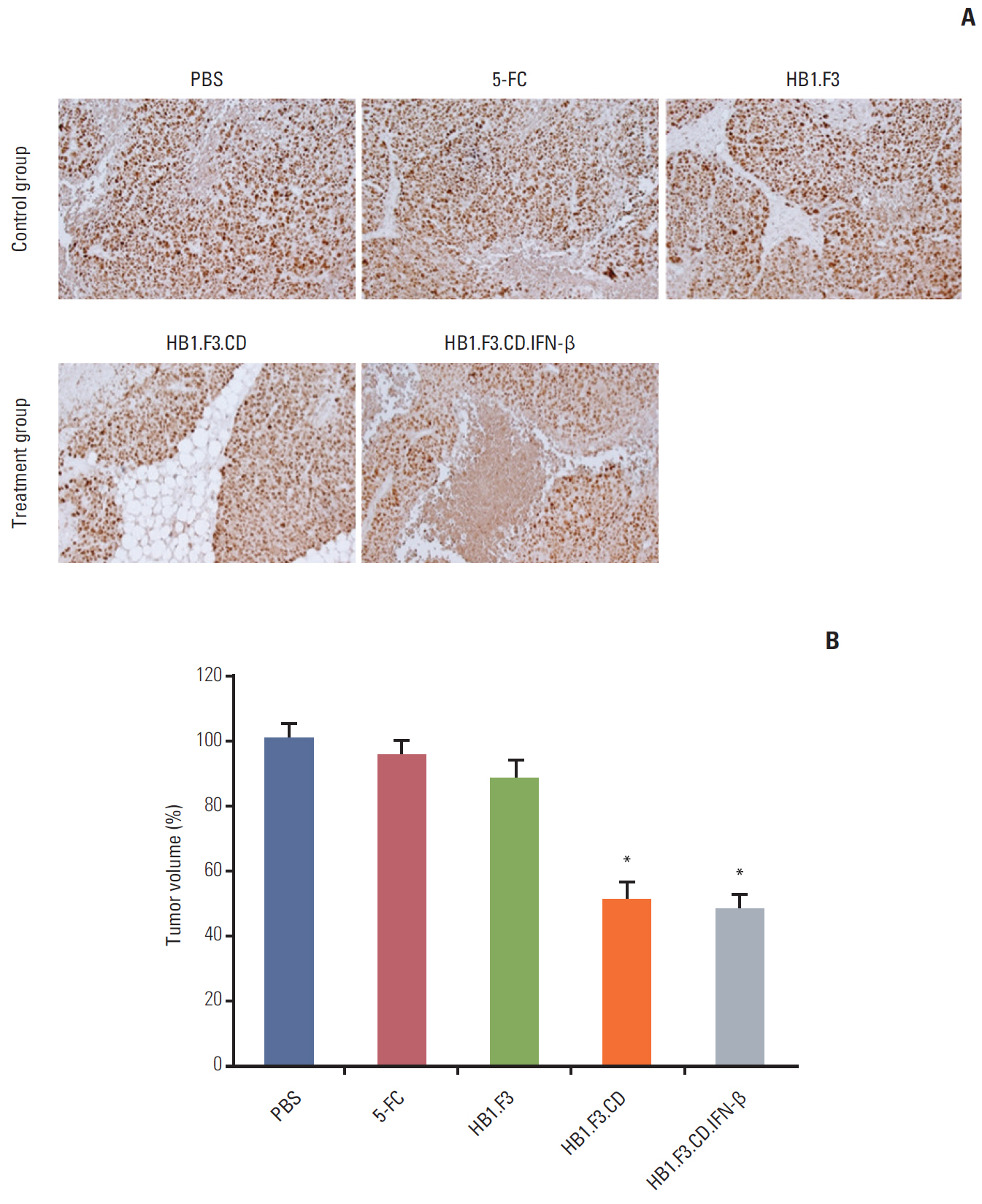
In results of a modified trans-well migration assay, HB1.F3.CD and HB1.F3.CD.IFN-β cells selectively migrated toward SW-620, human lymph node-derived metastatic colorectal adenocarcinoma cells. The viability of SW-620 cells was significantly reduced when co-cultured with HB1.F3.CD or HB1.F3.CD.IFN-β cells in the presence of 5-FC. In addition, it was found that the tumor-tropic properties of these engineered human neural stem cells (hNSCs) were attributed to chemoattractant molecules including stromal cell-derived factor 1, c-Kit, urokinase receptor, urokinase-type plasminogen activator, and C-C chemokine receptor type 2 secreted by SW-620 cells. In a xenograft mouse model, treatment with hNSC resulted in significantly inhibited growth of the tumor mass without virulent effects on the animals.
CONCLUSION
The current results indicate that engineered hNSCs and a prodrug treatment inhibited the growth of SW-620 cells. Therefore, hNSC therapy may be a clinically effective tool for the treatment of lymph node metastatic colorectal cancer.
MeSH Terms
-
Adenocarcinoma*
Animals
Chemokine CXCL12
Colorectal Neoplasms
Cytosine Deaminase*
Cytosine*
DNA
Escherichia coli
Flucytosine
Fluorouracil
Genetic Therapy
Heterografts*
Humans
Interferon-beta
Lymph Nodes
Lymphatic Metastasis
Mice*
Neural Stem Cells*
Polymerase Chain Reaction
Reverse Transcription
Stem Cells
Urokinase-Type Plasminogen Activator
Chemokine CXCL12
Cytosine
Cytosine Deaminase
DNA
Flucytosine
Fluorouracil
Interferon-beta
Urokinase-Type Plasminogen Activator
Figure
Reference
-
References
1. Galandiuk S, Wieand HS, Moertel CG, Cha SS, Fitzgibbons RJ Jr, Pemberton JH, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992; 174:27–32.2. Khattak MA, Martin HL, Beeke C, Price T, Carruthers S, Kim S, et al. Survival differences in patients with metastatic colorectal cancer and with single site metastatic disease at initial presentation: results from South Australian clinical registry for advanced colorectal cancer. Clin Colorectal Cancer. 2012; 11:247–54.
Article3. Dhar DK, Yoshimura H, Kinukawa N, Maruyama R, Tachibana M, Kohno H, et al. Metastatic lymph node size and colorectal cancer prognosis. J Am Coll Surg. 2005; 200:20–8.
Article4. Rahbari NN, Bork U, Motschall E, Thorlund K, Buchler MW, Koch M, et al. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2012; 30:60–70.
Article5. Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003; 10:65–71.
Article6. Yi BR, Hwang KA, Aboody KS, Jeung EB, Kim SU, Choi KC. Selective antitumor effect of neural stem cells expressing cytosine deaminase and interferon-beta against ductal breast cancer cells in cellular and xenograft models. Stem Cell Res. 2014; 12:36–48.
Article7. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003; 3:330–8.
Article8. Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002; 62:4968–76.9. Garrison JI, Berens ME, Shapiro JR, Treasurywala S, Floyd-Smith G. Interferon-beta inhibits proliferation and progression through S phase of the cell cycle in five glioma cell lines. J Neurooncol. 1996; 30:213–23.
Article10. Ren C, Kumar S, Chanda D, Kallman L, Chen J, Mountz JD, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008; 15:1446–53.11. Le Page C, Genin P, Baines MG, Hiscott J. Interferon activation and innate immunity. Rev Immunogenet. 2000; 2:374–86.12. Yi BR, Kim SU, Kim YB, Lee HJ, Cho MH, Choi KC. Antitumor effects of genetically engineered stem cells expressing yeast cytosine deaminase in lung cancer brain metastases via their tumor-tropic properties. Oncol Rep. 2012; 27:1823–8.
Article13. Kim SU, Nakagawa E, Hatori K, Nagai A, Lee MA, Bang JH. Production of immortalized human neural crest stem cells. Methods Mol Biol. 2002; 198:55–65.
Article14. Ito S, Natsume A, Shimato S, Ohno M, Kato T, Chansakul P, et al. Human neural stem cells transduced with IFN-beta and cytosine deaminase genes intensify bystander effect in experimental glioma. Cancer Gene Ther. 2010; 17:299–306.15. Yi BR, Kim SU, Choi KC. Co-treatment with therapeutic neural stem cells expressing carboxyl esterase and CPT-11 inhibit growth of primary and metastatic lung cancers in mice. Oncotarget. 2014; 5:12835–48.
Article16. Yi BR, Hwang KA, Kang NH, Kim SU, Jeung EB, Kim HC, et al. Synergistic effects of genetically engineered stem cells expressing cytosine deaminase and interferon-beta via their tumor tropism to selectively target human hepatocarcinoma cells. Cancer Gene Ther. 2012; 19:644–51.17. Yi BR, Kim SU, Choi KC. Synergistic effect of therapeutic stem cells expressing cytosine deaminase and interferon-beta via apoptotic pathway in the metastatic mouse model of breast cancer. Oncotarget. 2016; 7:5985–99.
Article18. Bockelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015; 54:5–16.19. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014; 383:1490–502.
Article20. Fulmes M, Setrakian S, Raj PK, Bogard BM. Cancer biology and necrotic changes in metastatic lymph nodes and survival of colon cancer patients. Am J Surg. 2005; 189:364–8.
Article21. Guyot F, Faivre J, Manfredi S, Meny B, Bonithon-Kopp C, Bouvier AM. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann Oncol. 2005; 16:756–61.
Article22. Yi C, Huang Y, Guo ZY, Wang SR. Antitumor effect of cytosine deaminase/5-fluorocytosine suicide gene therapy system mediated by Bifidobacterium infantis on melanoma. Acta Pharmacol Sin. 2005; 26:629–34.
Article23. Wang X, Ji C, Ma D, Zhao J, Hou M, Yu H, et al. Antitumor effects of cytosine deaminase and thymidine kinase fusion suicide gene under the control of mdr1 promoter in mdr1 positive leukemia cells. Leuk Lymphoma. 2007; 48:1600–9.
Article24. You MH, Kim WJ, Shim W, Lee SR, Lee G, Choi S, et al. Cytosine deaminase-producing human mesenchymal stem cells mediate an antitumor effect in a mouse xenograft model. J Gastroenterol Hepatol. 2009; 24:1393–400.
Article25. Yi BR, Hwang KA, Kim YB, Kim SU, Choi KC. Effects of genetically engineered stem cells expressing cytosine deaminase and interferon-beta or carboxyl esterase on the growth of LNCaP rrostate cancer cells. Int J Mol Sci. 2012; 13:12519–32.26. Yi BR, Park MA, Lee HR, Kang NH, Choi KJ, Kim SU, et al. Suppression of the growth of human colorectal cancer cells by therapeutic stem cells expressing cytosine deaminase and interferon-beta via their tumor-tropic effect in cellular and xenograft mouse models. Mol Oncol. 2013; 7:543–54.27. Nelson TJ, Martinez-Fernandez A, Yamada S, Ikeda Y, Perez-Terzic C, Terzic A. Induced pluripotent stem cells: advances to applications. Stem Cells Cloning. 2010; 3:29–37.28. Kucerova L, Skolekova S, Demkova L, Bohovic R, Matuskova M. Long-term efficiency of mesenchymal stromal cell-mediated CD-MSC/5FC therapy in human melanoma xenograft model. Gene Ther. 2014; 21:874–87.
Article29. Zhang T, Lee YW, Rui YF, Cheng TY, Jiang XH, Li G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2013; 4:70.
Article30. Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008; 15:739–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Potential Therapy Using Engineered Stem Cells Prevented Malignant Melanoma in Cellular and Xenograft Mouse Models
- Enhancement of Bystander Prostate Cancer Cell Killing by the Utilization of Bone Marrow Stromal Cells Genetically Engineered to Express Cytosine Deaminase
- Selective Delivery of a Therapeutic Gene for Treatment of Head and Neck Squamous Cell Carcinoma Using Human Neural Stem Cells
- Adenovirus-Mediated Toxic Gene Therapy Using Cytosine Deaminase and Osteocalcin Promoter for the Treatment of Prostate Cancer
- Stimulatory Anticancer Effect of Resveratrol Mediated by G Protein-Coupled Estrogen Receptor in Colorectal Cancer