Clin Exp Otorhinolaryngol.
2013 Sep;6(3):176-183.
Selective Delivery of a Therapeutic Gene for Treatment of Head and Neck Squamous Cell Carcinoma Using Human Neural Stem Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Dongguk University Ilsan Hospital, Goyang, Korea. otolarynx@duih.org
- 2Institute for Regenerative Medicine, Gachon University Gil Hospital, Incheon, Korea.
- 3Division of Neurology, Department of Medicine, UBC Hospital, University of British Columbia, Vancouver, BC, Canada.
Abstract
OBJECTIVES
Based on studies of the extensive tropism of neural stem cells (NSCs) toward malignant brain tumor, we hypothesized that NSCs could also target head and neck squamous cell carcinoma (HNSCC) and could be used as a cellular therapeutic delivery system.
METHODS
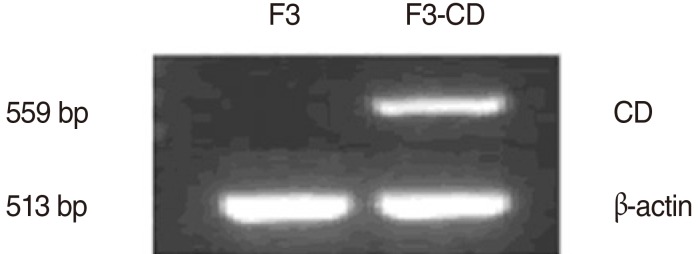
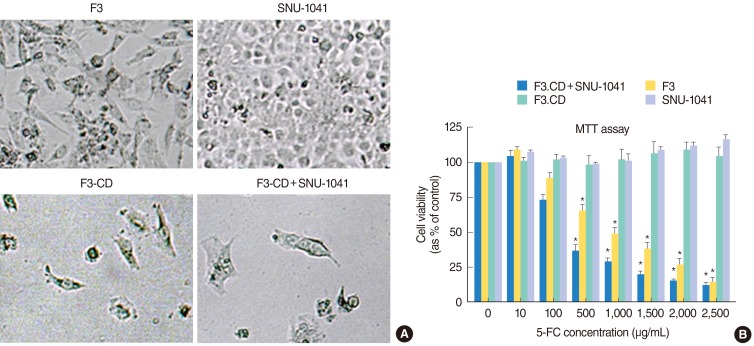
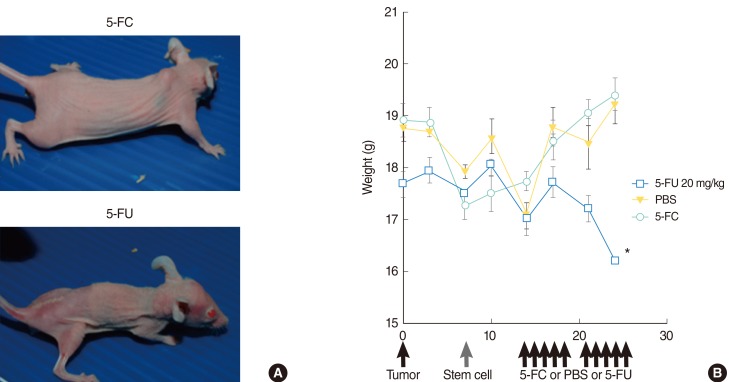
To apply this strategy to the treatment of HNSCC, we used a human NSC line expressing cytosine deaminase (HB1.F3-CD), an enzyme that converts 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU), an anticancer agent. HB1. F3-CD in combination with 5-FC were cocultured with the HNSCC (SNU-1041) to examine the cytotoxicity on target tumor cells in vitro. For in vivo studies, an HNSCC mouse model was created by subcutaneous implantation of human HNSCC cells into athymic nude mice. HB1.F3-CD cells were injected into mice using tumoral, peritumoral, or intravenous injections, followed by systemic 5-FC administration.
RESULTS
In vitro, the HB1.F3-CD cells significantly inhibited the growth of an HNSCC cell line in the presence of the 5-FC. Independent of the method of injection, the HB1.F3-CD cells migrated to the HNSCC tumor, causing a significant reduction in tumor volume. In comparison to 5-FU administration, HB1.F3-CD cell injection followed by 5-FC administration reduced systemic toxicity, but achieved the same level of therapeutic efficacy.
CONCLUSION
Transplantation of human NSCs that express the suicide enzyme cytosine deaminase combined with systemic administration of the prodrug 5-FC may be an effective regimen for the treatment of HNSCC.
Keyword
MeSH Terms
Figure
Reference
-
1. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004; 5. 350(19):1937–1944. PMID: 15128893.
Article2. Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol. 2004; 6. 22(11):2214–2232. PMID: 15169811.
Article3. Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007; 6. 67(13):6304–6313. PMID: 17616689.
Article4. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003; 5. 3(5):330–338. PMID: 12724731.
Article5. Hamada H, Kobune M, Nakamura K, Kawano Y, Kato K, Honmou O, et al. Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci. 2005; 3. 96(3):149–156. PMID: 15771617.
Article6. Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000; 11. 97(23):12846–12851. PMID: 11070094.
Article7. Aboody KS, Najbauer J, Schmidt NO, Yang W, Wu JK, Zhuge Y, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006; 4. 8(2):119–126. PMID: 16524944.8. Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004; 9. 24(3):159–171. PMID: 15484694.
Article9. Ourednik V, Ourednik J, Flax JD, Zawada WM, Hutt C, Yang C, et al. Segregation of human neural stem cells in the developing primate forebrain. Science. 2001; 9. 293(5536):1820–1824. PMID: 11474066.
Article10. Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998; 11. 16(11):1033–1039. PMID: 9831031.11. Cho T, Bae JH, Choi HB, Kim SS, McLarnon JG, Suh-Kim H, et al. Human neural stem cells: electrophysiological properties of voltage-gated ion channels. Neuroreport. 2002; 8. 13(11):1447–1452. PMID: 12167771.
Article12. Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005; 6. 7(6):623–629. PMID: 16036113.
Article13. Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003; 9. 34(9):2258–2263. PMID: 12881607.
Article14. Chu K, Kim M, Chae SH, Jeong SW, Kang KS, Jung KH, et al. Distribution and in situ proliferation patterns of intravenously injected immortalized human neural stem-like cells in rats with focal cerebral ischemia. Neurosci Res. 2004; 12. 50(4):459–465. PMID: 15567483.
Article15. Chu K, Kim M, Jeong SW, Kim SU, Yoon BW. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett. 2003; 6. 343(2):129–133. PMID: 12759181.
Article16. Ferrucci JT. Advances in abdominal MR imaging. Radiographics. 1998; Nov-Dec. 18(6):1569–1586. PMID: 9821200.
Article17. Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003; 8. 228(2):480–487. PMID: 12819345.
Article18. Bulte JW, Arbab AS, Douglas T, Frank JA. Preparation of magnetically labeled cells for cell tracking by magnetic resonance imaging. Methods Enzymol. 2004; 386:275–299. PMID: 15120257.
Article19. Kalish H, Arbab AS, Miller BR, Lewis BK, Zywicke HA, Bulte JW, et al. Combination of transfection agents and magnetic resonance contrast agents for cellular imaging: relationship between relaxivities, electrostatic forces, and chemical composition. Magn Reson Med. 2003; 8. 50(2):275–282. PMID: 12876703.
Article20. Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, et al. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003; 12. 229(3):838–846. PMID: 14657318.
Article21. Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, et al. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000; 8. 87(4):595–600. PMID: 10918203.
Article22. Shimato S, Natsume A, Takeuchi H, Wakabayashi T, Fujii M, Ito M, et al. Human neural stem cells target and deliver therapeutic gene to experimental leptomeningeal medulloblastoma. Gene Ther. 2007; 8. 14(15):1132–1142. PMID: 17508009.
Article23. Aboody KS, Bush RA, Garcia E, Metz MZ, Najbauer J, Justus KA, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS One. 2006; 12. 1:e23. PMID: 17183650.
Article24. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 1. 100(1):57–70. PMID: 10647931.
Article25. Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005; 4. 65(8):3307–3318. PMID: 15833864.
Article26. Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006; 9. 12(18):5550–5556. PMID: 17000692.
Article27. Mullen CA, Kilstrup M, Blaese RM. Transfer of the bacterial gene for cytosine deaminase to mammalian cells confers lethal sensitivity to 5-fluorocytosine: a negative selection system. Proc Natl Acad Sci U S A. 1992; 1. 89(1):33–37. PMID: 1729703.
Article28. Miller CR, Williams CR, Buchsbaum DJ, Gillespie GY. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002; 2. 62(3):773–780. PMID: 11830532.29. Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A. 1994; 8. 91(17):8302–8306. PMID: 8058798.
Article30. Xu G, McLeod HL. Strategies for enzyme/prodrug cancer therapy. Clin Cancer Res. 2001; 11. 7(11):3314–3324. PMID: 11705842.