Cancer Res Treat.
2019 Apr;51(2):797-811. 10.4143/crt.2018.364.
A Potential Therapy Using Engineered Stem Cells Prevented Malignant Melanoma in Cellular and Xenograft Mouse Models
- Affiliations
-
- 1Laboratory of Biochemistry and Immunology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. kchoi@cbu.ac.kr
- 2Department of Medicine, Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada.
- 3Institute of Life Science and Bio-Engineering, TheraCell Bio & Science, Cheongju, Korea.
- KMID: 2464425
- DOI: http://doi.org/10.4143/crt.2018.364
Abstract
- PURPOSE
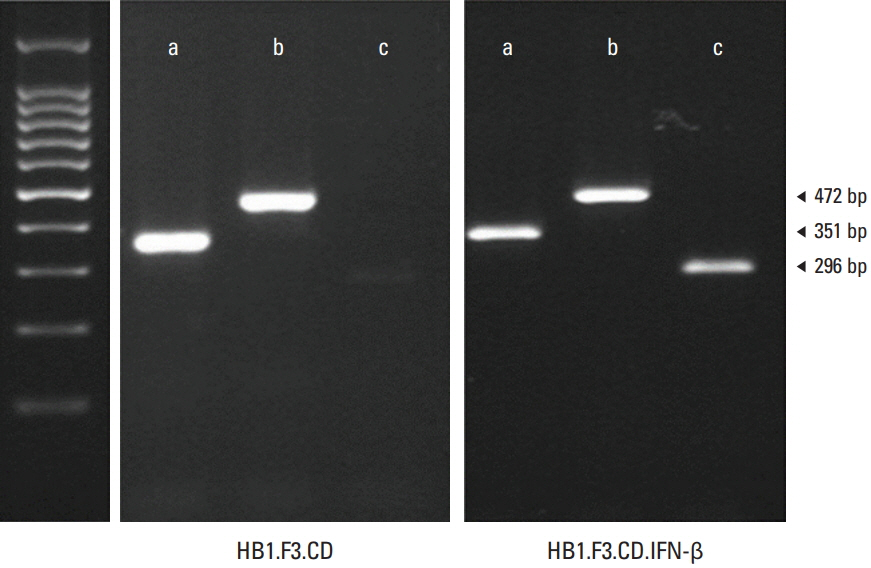
In the present study, human neural stem cells (hNSCs) with tumor-tropic behavior were used as drug delivery vehicle to selectively target melanoma. A hNSC line (HB1.F3) was transduced into two types: one expressed only the cytosine deaminase (CD) gene (HB1.F3. CD) and the other expressed both CD and human interferon-β (IFN-β) genes (HB1.F3.CD. IFN-β).
MATERIALS AND METHODS
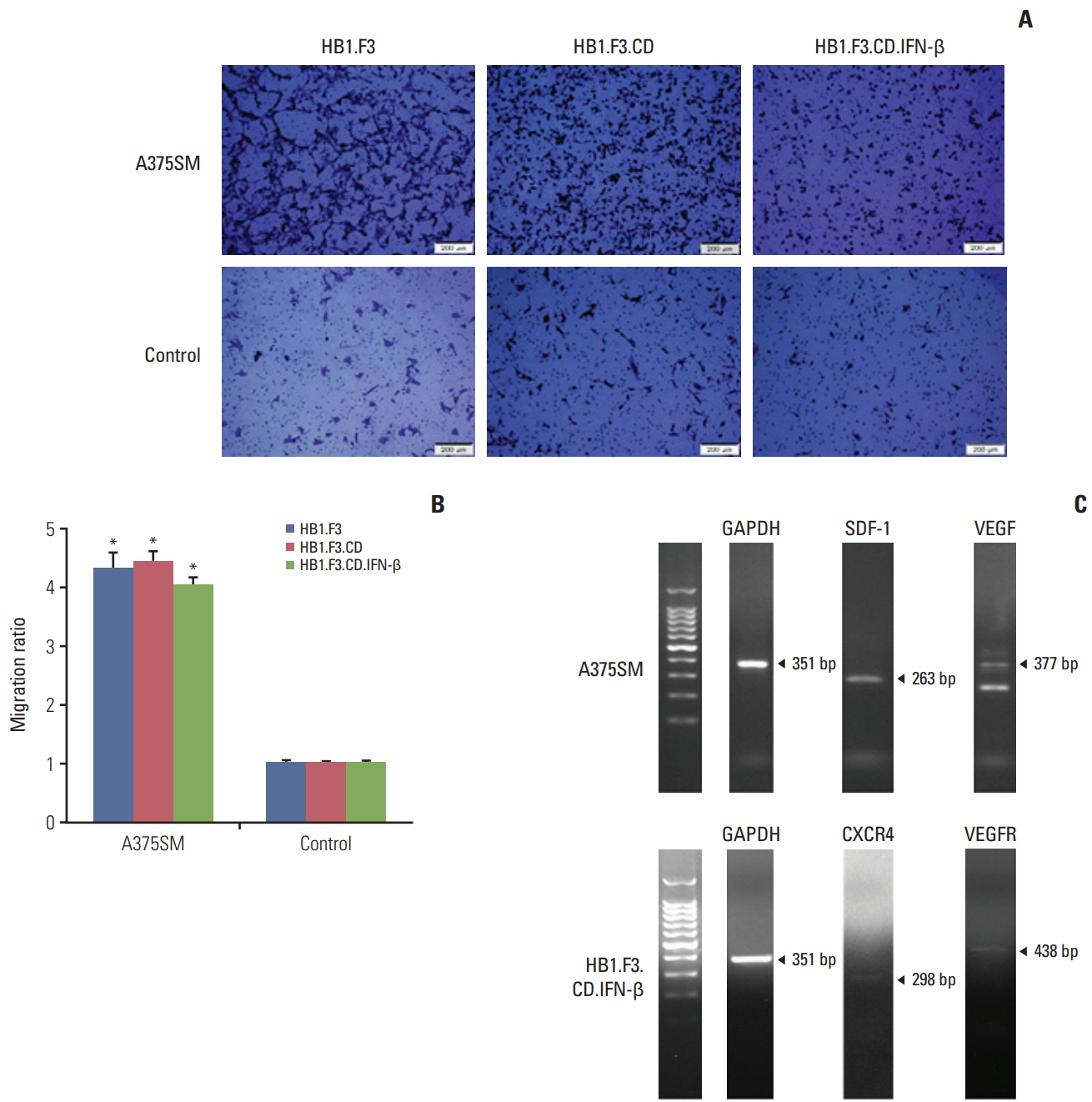
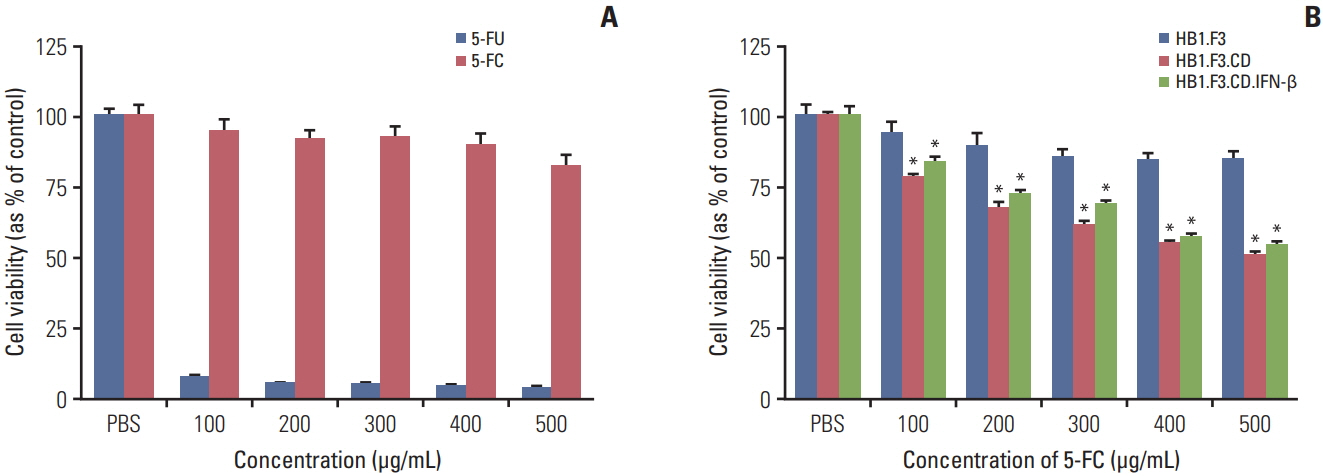
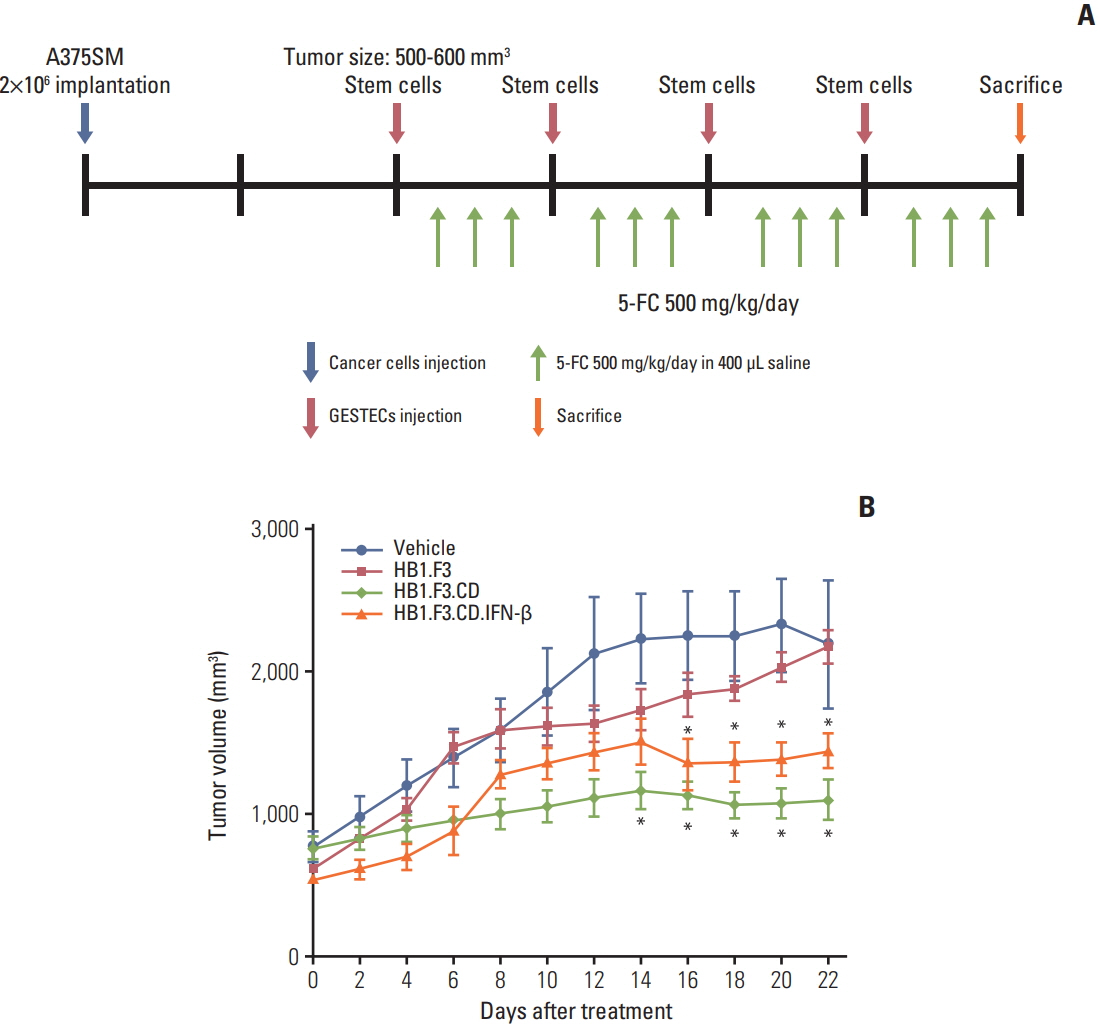
This study verified the tumor-tropic migratory competence of engineered hNSCs on melanoma (A375SM) using a modified Boyden chamber assay in vitro and CM-DiI staining in vivo. The antitumor effect of HB1.F3.CD and HB1.F3.CD.IFN-β on melanoma was also confirmed using an MTT assay in vitro and xenograft mouse models.
RESULTS
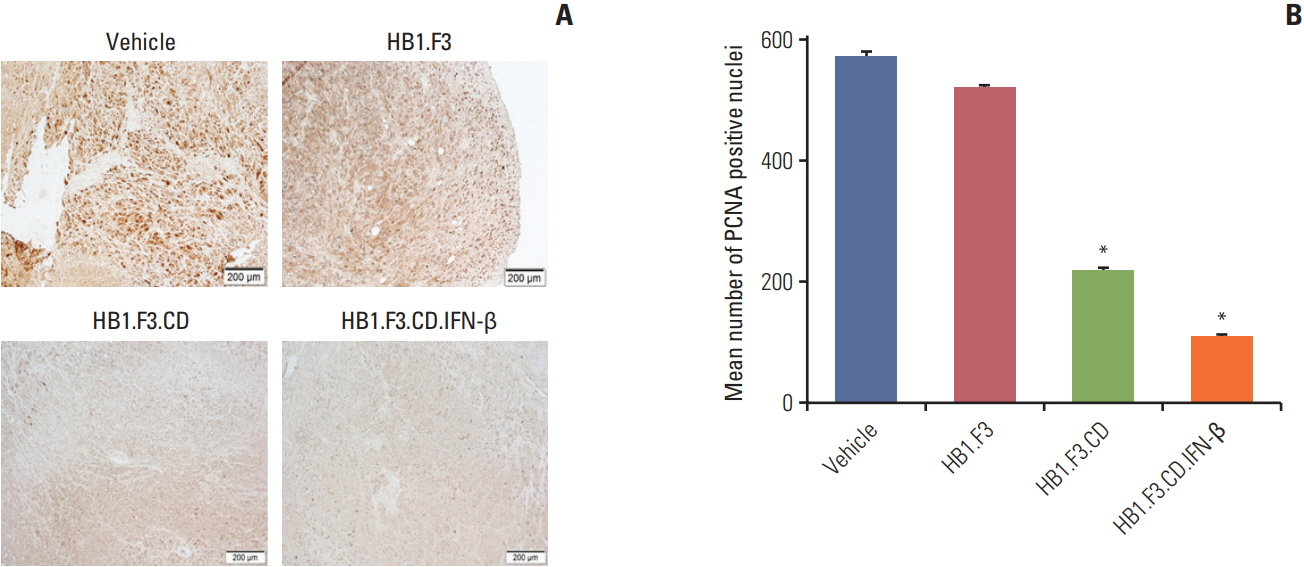
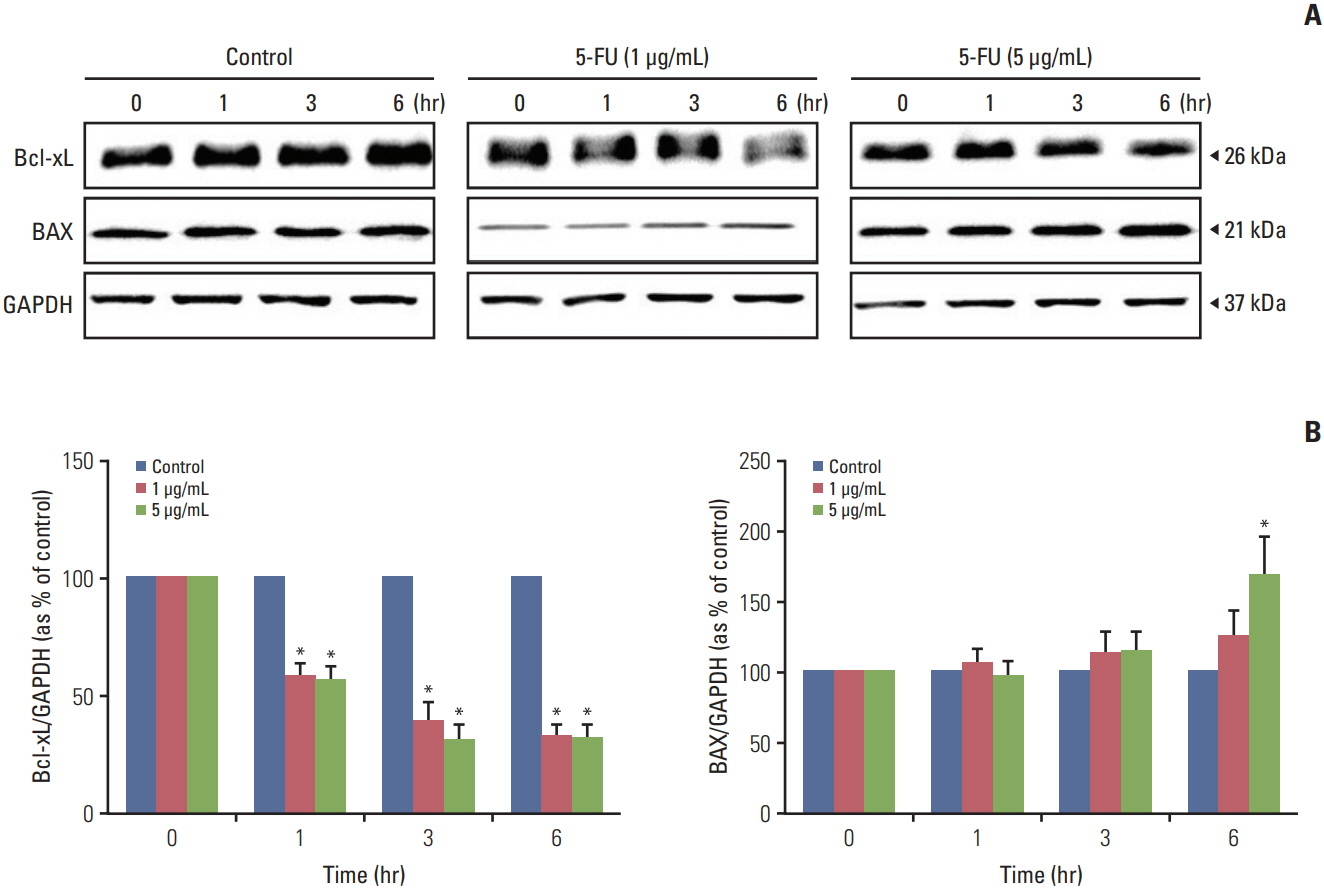
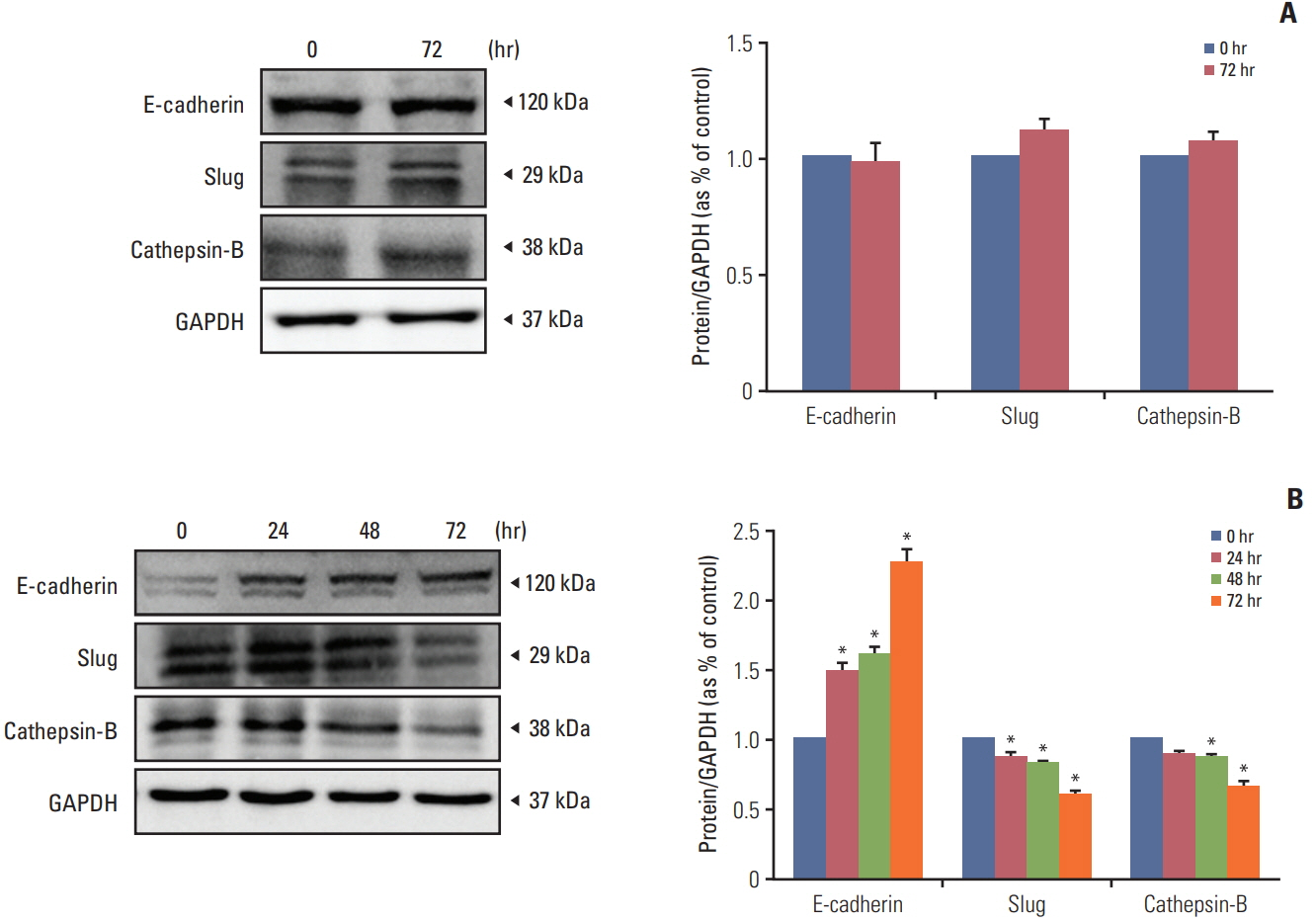
A secreted form of IFN-β from the HB1.F3.CD.IFN-β cells modified the epithelial-mesenchymal transition (EMT) process and metastasis of melanoma. 5-Fluorouracil treatment also accelerated the expression of the pro-apoptotic protein BAX and decelerated the expression of the anti-apoptotic protein Bcl-xL on melanoma cell line.
CONCLUSION
Our results illustrate that engineered hNSCs prevented malignant melanoma cells from proliferating in the presence of the prodrug, and the form that secreted IFN-β intervened in the EMT process and melanoma metastasis. Hence, neural stem cell-directed enzyme/prodrug therapy is a plausible treatment for malignant melanoma.
MeSH Terms
Figure
Reference
-
References
1. American Cancer Society. Cancer facts and figures 2017. Atlanta, GA: American Cancer Society;2017.2. Berwick M, Buller DB, Cust A, Gallagher R, Lee TK, Meyskens F, et al. Melanoma epidemiology and prevention. Cancer Treat Res. 2016; 167:17–49.
Article3. Russak JE, Rigel DS. Risk factors for the development of primary cutaneous melanoma. Dermatol Clin. 2012; 30:363–8.
Article4. Marzagalli M, Montagnani Marelli M, Casati L, Fontana F, Moretti RM, Limonta P. Estrogen receptor beta in melanoma: from molecular insights to potential clinical utility. Front Endocrinol (Lausanne). 2016; 7:140.
Article5. Han J, Shi C, Dong X, Wang J, Wen H, Wang B, et al. Laparoscopic abdomino-perineal resection for patients with anorectal malignant melanoma: a report of 4 cases. J Biomed Res. 2016; 30:436–40.
Article6. Wellbrock C, Arozarena I. The complexity of the ERK/MAPkinase pathway and the treatment of melanoma skin cancer. Front Cell Dev Biol. 2016; 4:33.
Article7. Flaherty KT, McArthur G. BRAF, a target in melanoma: implications for solid tumor drug development. Cancer. 2010; 116:4902–13.8. Funck-Brentano E, Helias-Rodzewicz Z, Longvert C, Mokhtari K, Saiag P, Emile JF. Increase in NRAS mutant allele percentage during metastatic melanoma progression. Exp Dermatol. 2016; 25:472–4.9. Kim T, Amaria RN, Spencer C, Reuben A, Cooper ZA, Wargo JA. Combining targeted therapy and immune checkpoint inhibitors in the treatment of metastatic melanoma. Cancer Biol Med. 2014; 11:237–46.10. Yi BR, Park MA, Lee HR, Kang NH, Choi KJ, Kim SU, et al. Suppression of the growth of human colorectal cancer cells by therapeutic stem cells expressing cytosine deaminase and interferon-beta via their tumor-tropic effect in cellular and xenograft mouse models. Mol Oncol. 2013; 7:543–54.11. Yi BR, Hwang KA, Kang NH, Kim SU, Jeung EB, Kim HC, et al. Synergistic effects of genetically engineered stem cells expressing cytosine deaminase and interferon-beta via their tumor tropism to selectively target human hepatocarcinoma cells. Cancer Gene Ther. 2012; 19:644–51.12. Hernandez-Alcoceba R, Sangro B, Prieto J. Gene therapy of liver cancer. World J Gastroenterol. 2006; 12:6085–97.
Article13. Yi BR, O SN, Kang NH, Hwang KA, Kim SU, Jeung EB, et al. Genetically engineered stem cells expressing cytosine deaminase and interferon-beta migrate to human lung cancer cells and have potentially therapeutic anti-tumor effects. Int J Oncol. 2011; 39:833–9.14. Garrison JI, Berens ME, Shapiro JR, Treasurywala S, Floyd-Smith G. Interferon-beta inhibits proliferation and progression through S phase of the cell cycle in five glioma cell lines. J Neurooncol. 1996; 30:213–23.
Article15. Ren C, Kumar S, Chanda D, Kallman L, Chen J, Mountz JD, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008; 15:1446–53.16. Ito S, Natsume A, Shimato S, Ohno M, Kato T, Chansakul P, et al. Human neural stem cells transduced with IFN-beta and cytosine deaminase genes intensify bystander effect in experimental glioma. Cancer Gene Ther. 2010; 17:299–306.17. Yi BR, Kim SU, Choi KC. Co-treatment with therapeutic neural stem cells expressing carboxyl esterase and CPT-11 inhibit growth of primary and metastatic lung cancers in mice. Oncotarget. 2014; 5:12835–48.
Article18. Aboody KS, Najbauer J, Danks MK. Stem and progenitor cellmediated tumor selective gene therapy. Gene Ther. 2008; 15:739–52.
Article19. Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004; 101:18117–22.20. Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005; 7:623–9.
Article21. Park GT, Kim SU, Choi KC. Anti-proliferative effect of engineered neural stem cells expressing cytosine deaminase and interferon-beta against lymph node-derived metastatic colorectal adenocarcinoma in cellular and xenograft mouse models. Cancer Res Treat. 2017; 49:79–91.22. Yi BR, Hwang KA, Aboody KS, Jeung EB, Kim SU, Choi KC. Selective antitumor effect of neural stem cells expressing cytosine deaminase and interferon-beta against ductal breast cancer cells in cellular and xenograft models. Stem Cell Res. 2014; 12:36–48.
Article23. Meng WC, Pan Y, Zhao X. Epirubicin-gold nanoparticles suppress hepatocellular carcinoma xenograft growth in nude mice. J Biomed Res. 2015; 29:486–90.
Article24. Mullen CA, Coale MM, Lowe R, Blaese RM. Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 1994; 54:1503–6.25. Heo JR, Kim NH, Cho J, Choi KC. Current treatments for advanced melanoma and introduction of a promising novel gene therapy for melanoma (Review). Oncol Rep. 2016; 36:1779–86.
Article26. Bredholt G, Mannelqvist M, Stefansson IM, Birkeland E, Bo TH, Oyan AM, et al. Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget. 2015; 6:39676–91.
Article27. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003; 3:330–8.
Article28. Yi BR, Kim SU, Choi KC. Synergistic effect of therapeutic stem cells expressing cytosine deaminase and interferon-beta via apoptotic pathway in the metastatic mouse model of breast cancer. Oncotarget. 2016; 7:5985–99.
Article29. Yi BR, Kim SU, Choi KC. Additional effects of engineered stem cells expressing a therapeutic gene and interferon-beta in a xenograft mouse model of endometrial cancer. Int J Oncol. 2015; 47:171–8.30. Kudryavets YI, Bezdenezhnykh NO, Lykhova OO, Semesiuk NI, Vorontsova AL. The role of interferon as a modifier of epithelial-mesenchymal transition in tumor cells. Exp Oncol. 2011; 33:178–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-proliferative Effect of Engineered Neural Stem Cells Expressing Cytosine Deaminase and Interferon-β against Lymph Node–Derived Metastatic Colorectal Adenocarcinoma in Cellular and Xenograft Mouse Models
- Targeting Orthotopic Glioma in Mice with Genetically Engineered Salmonella typhimurium
- Current Concepts of Stem Cell Therapy
- Combination Therapy for Gliomas Using Temozolomide and Interferon-Beta Secreting Human Bone Marrow Derived Mesenchymal Stem Cells
- Glutathione Dynamics in the Tumor Microenvironment: A Potential Target of Cancer Stem Cells and T Cells